Abstract
The specific removal of bilirubin is essential to cure hyperbilirubinemia. In this study, cellulose membranes were prepared by a phase inversion technique with surface functionalized Procion Blue H-5R (PB) for bilirubin adsorption. The chemical structures and morphologies of membranes were characterized by FITR, XPS and SEM. The immobilized PB amounts of membranes were investigated. Moreover the effects of immobilized PB amounts, initial concentration of bilirubin, NaCl concentration, pH value, recycle numbers and BSA concentration on adsorption capability of PB-immobilized cellulose membrane to bilirubin. Results showed that PB was immobilized onto the cellulose membrane surfaces and reached a maximum amount of 230 mg/g membrane. The bilirubin adsorption isotherm fitted Langmuir model well. Furthermore, the PB-immobilized membrane could specifically adsorb bilirubin in BSA/bilirubin mixed solution and bilirubin adsorption capacity reached 26 mg/g at pH 9.5. The membrane showed excellent reusability that the adsorption capability of bilirubin decreased 1.3% after 3 adsorption–desorption cycles. This work indicated that the Procion Blue H-5R can be expected as a ligand for specific removal of bilirubin.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The removal of endogenous toxins is of great value to prolong longevity and improve healthy level. Bilirubin, a degradation product of hemoglobin, is one of endogenous toxins (Ostrow et al. 1994). Once the concentration of bilirubin is far over 17.1 umol/L in blood, it will cause irreversible injury of nervous system and even death (Ma et al. 2017a), especially in infant. There are many techniques applying in removing bilirubin, like phototherapy, molecular adsorbent recirculating system and hemoperfusion (Ma et al. 2017b). Among them, hemoperfusion is the highest efficient way to remove bilirubin in blood. Its nature of binding with albumin increases definitely obstacles for the selective removal of bilirubin (Avramescu et al. 2004). Therefore, it is urgent to find an adsorbent that has the specific adsorption to bilirubin.
The mesoporous carbon spheres were prepared for the removal of bilirubin by size selection (Guo et al. 2009, 2010; Liu et al. 2014). Albumin was still adsorbed on the sphere surface because of its strong surface tension. Moreover, Chen et al. prepared nano-CaCO3/polystyrene nanocomposite beads to remove bilirubin (Chen et al. 2018). The beads had an excellent adsorption performance in human plasma but low specific adsorption to bilirubin. Recently, the researchers have been focused on some specific ligands, such as amine (Jiang et al. 2017), hydroxyl (Wei et al. 2012) and Cibacron Blue F3GA (Xia et al. 2003), which adsorbed bilirubin via electrostatic interaction, hydrogen bond and hydrophobic interaction respectively. For instance, amine-functionalized PVA-co-PE nanofibrous membrane (Wang et al. 2017) was used to adsorb bilirubin from solution containing BSA, which showed 35 mg/g membrane of bilirubin adsorption capacity in 40 g/L BSA solution. However, some human proteins also can be adsorbed onto the amine-functionalized membrane through electrostatic interaction, which resulted in the low specific adsorption to bilirubin. In addition, bilirubin was absorbed onto Cibacron Blue F3GA (CB) immobilized poly (hydroxyethylmethacrylate-co-glycydylmethacrylate) membranes via the hydrophobic interaction (Bayramoglu et al. 2005). Altintas et al. prepared CB-attached poly (glycidyl methacrylate) (PGMA) beads for bilirubin removal from human serum, which showed specific adsorption to bilirubin (Altintas et al. 2011). To further study the binding mechanism of hydrophobic interaction, Liang-Schenkelberg et al. found aromatic group of CB can bind with hydrophobic parts of proteins by using molecular dynamics simulations (Liang-Schenkelberg et al. 2017). Three-dimensionally porous graphene was prepared as an efficient absorbent of bilirubin due to π-conjugated part of bilirubin, and bilirubin was absorbed onto porous graphene by π-π stacking (Ma et al. 2017a). Besides, Tao et al. proved that bilirubin can be removed selectively via π-π interaction (Tao et al. 2014). In recent years, dyes affinity membrane as a promising membrane applied for separation of biomolecules has attracted much attention. Protein, albumin, enzyme and bilirubin could be adsorbed by immobilizing different dye ligands such as CB (Dou et al. 2018; Wang et al. 2018), Reactive Orange 4 dye (Ng et al. 2019), phenol red (Sochacka 2015) onto membranes, microspheres or other carriers. Moreover, the surface mobilized dyes affinity ligands allowed the membranes to become more efficient and specific in capturing target biomolecules.
As a biocompatible material, cellulose membrane is widely used to introduce functional groups in biological separation field (Tamahkar et al. 2010; Ma et al. 2005; Lu and Hsieh 2010). In this work, we tried to use a new functional group, Procion Blue H-5R (PB), to prepare a new absorbent for specific removal of bilirubin. PB, one of the triazine dyes, could be used as affinity ligand to adsorb bilirubin in bilirubin separation. PB can be easily immobilized onto the hydroxyl group of cellulose membrane by the nucleophilic substitution reaction. PB had high affinity for bilirubin to be adsorbed on support surface closely due to hydrophobic, hydrogen bonding and electrostatic interactions. π-π interaction in hydrophobic interaction play a leading role in this adsorption process. Therefore, PB was chose to modify cellulose membrane. The cellulose membrane was prepared by a phase inversion technique. Procion Blue H-5R molecules were immobilized on cellulose membrane in alkali condition. The adsorption capacity of modified cellulose membranes to bilirubin was studied. Furthermore, the adsorption kinetics and isotherms for bilirubin were studied. PB-immobilized cellulose membrane combined adsorption and separation in one process and could be applied for hemoperfusion as an efficient adsorptive membrane. Besides, it could be applied for water purification to absorb some hydrophobic pollutant through π-π interaction.
Experimental
Materials
Sodium hydroxide (NaOH), thiourea (CH4N2S), polyethylene glycol (PEG) 2000 and sodium chloride (NaCl) were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Microcrystalline cellulose (molecular weight: 36,000) was provided from Fred Biological Technology Co. Ltd. (Suzhou, China). Procion Blue H-5R (PB) was obtained from Shanghai Annoqi Co. Ltd (Shanghai, China). Bilirubin was purchased from Minglang Biological Technology Co. Ltd. (Xian, China). Bovine serum albumin (BSA) were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Deionized water was used throughout the studies.
Preparation of cellulose membrane
The cellulose membrane was prepared as following procedure. Briefly, 4.51 g of microcrystalline cellulose and 0.63 g of PEG were added to 60 g aqueous alkaline solution (NaOH: CH4N2S: H2O = 10:8:82 (w/w/w)) which was frozen for 1 h in −24 °C refrigerator, and stirred vigorously for 10 min at room temperature. Then the above solution was centrifuged at 8000 rpm for 5 min to obtain a transparent solution containing 7 wt% cellulose and 1 wt% PEG. Afterwards, the resulting solution was immediately cast on a glass plate to give thickness of 200 um and immersed into deionized water to coagulate. The resulting cellulose membrane was washed with deionized water and was air-dried at ambient temperature for the characterization.
Procion Blue H-5R immobilization on cellulose membrane
Procion Blue H-5R molecules can be covalently attached to cellulose membrane surface according to Scheme 1. 45 mg PB was dissolved in 150 mL water, and the solution was heated to 60 °C, followed by addition of 50 mg cellulose membrane. After immersing for 10 min, 17.55 g NaCl was added into the solution. The temperature was maintained at 60 °C for 30 min, and then increased to 80 °C for 2 h. During the course, pH value of this solution was adjusted by adding certain amount of 0.1 M NaOH. The modified cellulose membranes were washed thoroughly by water until no PB molecule in the washing solution can be detected by measurement of UV–vis absorbance. Subsequently, residual solution in the reaction vessel and the washing solution were carefully collected together, measured by a UV–vis spectrophotometer at 570 nm to determine the amounts of PB that was not immobilized on cellulose membrane. A standard calibration curve was established to calculate PB concentration in solution. PB immobilization amount on cellulose membrane was determined by the difference of initial and final PB amounts in solution.
Characterization of membranes
The chemical structures of membranes were investigated by Fourier Transform infrared spectroscopy (FTIR, NEXUS-670) and X ray Photoelectron Spectroscopy (XPS, Escalab 250Xi). Morphologies of membranes were observed by using a scanning electron microscopy (SEM, Quanta 250). The concentration of bilirubin in PB solution was measured on a UV–Vis spectrophotometer (UV-1800).
Bilirubin adsorption studies
Bilirubin was firstly dissolved in 0.1 M NaOH solution and then diluted with phosphate buffer (PBS, pH = 7.4) to 200 mg/L. Because bilirubin is easily destroyed by exposure to direct sunlight and oxidized in alkaline solution, all adsorption experiments were carried out in a dark room, simultaneously followed with a control sample. In addition, the bilirubin concentration was detected by UV–Vis spectrophotometer at 438 nm and the one containing bilirubin-BSA complex at 460 nm. The adsorption experiments were all carried out at 37 °C.
The membranes with different PB immobilization amounts (10 mg) were immersed into 12 mL bilirubin solution respectively. Bilirubin adsorption capacity was determined with Eq. (1):
where q is adsorption capacity of PB immobilized membrane (mg/g); cb and ct are the bilirubin concentration of control solution and bilirubin solution after adsorption, respectively (mg/L); V is the volume of bilirubin solution (L); m is the mass of membrane (g). The bilirubin concentration was detected after adsorption for 0.5 h, 1 h, 2 h, 3 h, and 4 h.
Furthermore, bilirubin adsorption capacities of the PB-immobilized membrane were measured under different initial bilirubin concentrations, BSA concentrations, pH values, ionic strengths and recycle numbers.
Results and discussion
PB immobilization on cellulose membrane
Characterization of PB immobilized cellulose membranes
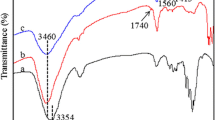
The chemical structural changes of membranes were observed by FTIR and XPS, and the results are shown respectively in Fig. 1a, b. In Fig. 1a(c), two main characteristic adsorption peaks on the PB-immobilized membrane, 1557 cm−1 and 1261 cm−1, are attributed to the aromatic ring and C–N stretching vibrations from PB molecule, respectively (Xia et al. 2003; Zhu et al. 2011). The C–O–C bond formed from the nucleophilic reaction of the PB triazinyl chloride with the cellulose hydroxyl overlapped with C–O–C vibration at 1070 cm−1 and could not be clearly discerned. Compared to original cellulose membrane [Fig. 1a, (b)], the presence of PB molecules immobilized on the membranes was confirmed by these two characteristic adsorption peaks. From Fig. 1b, XPS spectra of both original and PB-immobilized membranes showed the C 1 s region at 284 eV (Wang et al. 2017). However, the peak at 166 eV appeared in PB-immobilized membranes, which was corresponded to the S 2 p region. For indicating intuitively changes of surface chemistry, the elements (C, S and N) contents were showed in Table 1. In summary, it confirmed that PB molecules were immobilized on the cellulose membrane surface.
Morphology of PB immobilized cellulose membranes
To investigate the morphology changes of cellulose membrane, the SEM images of top, bottom surfaces and cross section of cellulose membrane and PB-immobilized cellulose membrane are shown in Fig. 2. It can be seen that the surface of cellulose membrane showed dense structure with spherical particles covering (Fig. 2a1 and a2). The surface structure was destroyed severely by NaOH and the number of spherical particles decreased considerably after PB was immobilized on membrane, which led to specific surface area. Besides, PB-immobilized cellulose membrane had rougher and looser cross section compared to cellulose membrane.
PB immobilization amounts on cellulose membrane
Being similar to Cibacron blue F3GA (CB) dye (Liang-Schenkelberg et al. 2017), PB also contains a naphthoquinone portion that has capacity of binding π-conjugated parts of bilirubin. Simultaneously, PB can be covalently immobilized onto the cellulose membrane surface via nucleophilic reactions between PB chloride and reactive hydroxyl groups on the cellulose.
PB was immobilized onto cellulose membrane as following procedure. PB molecules were adsorbed physically onto the membrane surface firstly, then attached covalently to the hydroxyl groups of the cellulose. Therefore, ionic strength of the solution has a crucial impact on PB immobilization amounts. As shown in Fig. 3a, the amount of PB immobilization was merely 2 mg/g membrane in the absence of NaCl. The amount of PB immobilized on membrane increased with increasing the concentration of NaCl in the solution. As an electrolyte, NaCl could weaken the electric repulsion between the negative charged PB molecules and hydroxyl groups on the membrane surfaces (Zhu et al. 2011). Thus, 2 mol/L NaCl was chose in following PB immobilization. More alkaline condition could promote the nucleophilic reaction between hydroxyl and chloride groups. As shown in Fig. 3b, the amounts of PB immobilization increased with increasing pH value. However, cellulose membrane surface was etched violently at extreme alkaline condition. Therefore, pH 11 was selected for further PB immobilization. As shown in Fig. 3c, the amounts of PB immobilized on cellulose membranes reached 230 mg/g membrane. At the stage of previous 30 min, the amount of PB immobilization increased rapidly and was effected deeply by the initial PB concentration, which is maybe a result of physical diffusion. It is likely to form multiple layer PB adsorption on the membrane (Lu and Hsieh 2009). After that, PB molecules began to attach covalently to cellulose membrane surface and finally reached equilibrium. PB immobilization amount was controlled by adjusting PB concentration in the following bilirubin adsorption studies. Besides, the stability of immobilized PB molecules on the membranes was studied by immersing PB-immobilized membranes in NaOH solution with different pH values (pH 7, 9, 10 and 11) for 1 day. PB molecules were not detected in the solution.
Bilirubin adsorption on PB-immobilized membrane
Effects of PB immobilization amounts, BSA concentration, ionic strength and pH value on bilirubin adsorption capacity
The cellulose membranes with different PB immobilization amounts were prepared by changing PB concentration of 0.1 g/L, 0.2 g/L, 0.3 g/L as mention above, and the PB-immobilized membranes were used to adsorb bilirubin from PBS buffer. The adsorption capacity versus time of different PB-immobilized membranes are shown in Fig. 4a. From Fig. 4a, it can be seen that the bilirubin adsorption capacity of original cellulose membrane was 2.5 mg/g membrane and almost unchanged with extending time, which was attributable to the nonspecific adsorption. However, the bilirubin adsorption capacity of PB-immobilized membranes was much higher than that of original membrane. It may be a result of the π-π interaction between PB benzene groups on the surface of PB-immobilized membranes and bilirubin molecules (Tao et al. 2014; Peng et al. 2016). To some extent, bilirubin adsorption capacity depends on the amounts of the functional groups on membrane. Therefore, the adsorption capacity of PB-immobilized membrane increased with increasing PB concentration, and reached the highest (22.7 mg/g membrane) when PB concentration was 0.3 g/L. It means that more PB molecules were immobilized on the membranes with increasing PB concentration. Therefore the PB-immobilized membrane treated with 0.3 g/L PB was chosen to be investigated further. As reported, bilirubin binds with albumin easily forms a bilirubin-albumin complex in the blood. It is of great value to study the specific bilirubin adsorption in presence of albumin. BSA was used to study the effect of albumin on bilirubin adsorption since BSA is similar to albumin in molecular structure and easier to gain comparison to albumin (Ma et al. 2017a).
The bilirubin adsorption capacity of different PB-functionalized cellulose membranes versus time (a) and effect of BSA concentration (b), NaCl concentration (c) (pH 11) and pH value (d) (NaCl concentration: 0 M) on bilirubin adsorption capacity of PB-functionalized cellulose membranes (bilirubin concentration is 200 mg/L and the adsorption time is 3 h)
The effect of BSA concentrations on bilirubin adsorption capacity of PB-immobilized membrane was studied, and the results can be seen in Fig. 4b. As shown in Fig. 4b, the bilirubin adsorption capacity of PB-immobilized membrane decreased rapidly with the BSA concentration increased firstly. When BSA concentration was higher than 2.5 g/L, bilirubin adsorption capacity of PB-immobilized membrane was constant, 4 mg/g. According to previous literatures (Moosavi-Movahedi et al. 2007; Wang et al. 2017), it is confirmed that there is the electrostatic interaction between bilirubin and BSA and each BSA molecule has 12 binding sites for bilirubin. The free bilirubin (unbound with BSA) was dominant in solution when BSA concentration was lower than 2.5 g/L. BSA has a larger molecular volume compared to bilirubin molecule, which hinders the contact of bilirubin with the ligand molecules (Zhang and Gu 2006). Therefore, it was speculated that the bilirubin adsorption of PB-immobilized membrane was easily affected by the steric hindrance than interaction between bilirubin and BSA. The amount of free bilirubin reached the lowest value and kept stable when BSA concentration was more than 2.5 g/L, which led to low bilirubin adsorption capacity.
The effect of ionic strength on bilirubin adsorption capacity was investigated, as shown in Fig. 4c. From Fig. 4c, it can be seen that the bilirubin adsorption capacity decreased slightly with NaCl concentration increased. This result is consistent with others (Jiang et al. 2017; Wang et al. 2017). It may be explained that the addition of NaCl deprotonates the sulfonate groups of PB molecules (Zhu et al. 2011), which results in repulsively negative carboxyl ion of bilirubin in the experimental conditions. However, the π-π interaction between PB and bilirubin molecules played a dominant role so that high ionic strength led to slight decreased bilirubin adsorption. Figure 4d showed the effect of pH value on bilirubin adsorption capacity. Since bilirubin did not dissolve in neutral solution, the experimental was carried out in alkaline solution. The bilirubin adsorption capacity decreased gradually with increasing pH value from 9 to 11. As reported before, there is an electrostatic repulsion between the carboxyl of bilirubin and the sulfonate groups of PB molecules at high pH value. The negative charge of carboxyl increased with increasing pH value, which led to stronger repulsion. The results can also be explained by another reason. Bilirubin molecule was wrapped by hydrophobic groups because of intact inner hydrogen bond, which was beneficial to form the π-π interaction (Xia et al. 2003). This structure would be destroyed when pH value increased. Therefore, high pH value has a negative influence on bilirubin adsorption.
Bilirubin adsorption equilibrium
The effect of initial bilirubin concentration on adsorption capacity of PB-immobilized membrane was investigated, and the results were showed in Fig. 5a. From Fig. 5a, the adsorption capacity increased rapidly with the bilirubin concentration increased and tended to be constant at 200 mg/L. The adsorption capacity of bilirubin reached around 25 mg/g membrane when the initial bilirubin concentration was 400 mg/L.
In the design of adsorption process, the adsorption isotherm plays a significant role, which can clearly describe the interactive form between substance and the adsorbent. Therefore, it is necessary to build an adsorption model. In this work, the adsorption process of bilirubin through Langmuir and Freundlich adsorption isotherms was studied.
In the Langmuir model, it is usually hypothesized that the adsorbed molecules are independent, molecules is adsorbed on surface in monolayer and every interactive site has equal adsorption ability (Jiang et al. 2017). The Langmuir equation can be expressed in the Eq. (2) (Jiang et al. 2017; Wang et al. 2017):
where ce (mg/L) is the equilibrium bilirubin concentration; qe(mg/g) is the bilirubin adsorption capacity at equilibrium; qmax (mg/g) is the maximum adsorption capacity and K is the Langmuir adsorption constant.
The Langmuir adsorption isotherm of PB-immobilized membrane is presented in Fig. 5b, and the correlation coefficients of the Langmuir equation is showed in Table 2. As shown in the Table 2, the correlation coefficient of the Langmuir equation (r2) is 0.967, which means that the bilirubin adsorption of PB-immobilized membrane fits basically with the Langmuir adsorption isotherm. It denoted that this adsorption is a monolayer adsorption process. In this work, bilirubin was oxidized in the alkaline solution, and therefore there might be a bit error in fitting Langmuir adsorption isotherm.
The Freundlich isotherm adsorption is an empirical model, which is established from experimental data, and could be expressed in the Eq. (3) (Jiang et al. 2017):
where K is the Freundlich adsorption constant; 1/n is a factor; qe (mg/g) and ce (mg/L) is the same as those in the Langmuir equation.
The Freundlich adsorption isotherm of PB-immobilized membrane is presented in Fig. 5c, and the correlation coefficient of the Freundlich equation is showed in Table 2. As shown in Table 2, the correlation coefficient of the Freundlich equation (r2) is far lower than that of Langmuir equation. Thus, the Langmuir model is more suitable to depict the bilirubin adsorption process of PB-immobilized membrane than the Freundlich model.
Bilirubin recovery of PB-immobilized membrane
The recovery as an important adsorption performance was also investigated and the result showed in Fig. 6. As shown in Fig. 6, the bilirubin adsorption capacity of PB-immobilized membrane barely decreased after 3 recycles and the desorption efficiency (desorption/adsorption) was still kept at 86.5% on third time, which indicated that PB-immobilized membranes had an excellent recovery ability. In addition, the bilirubin adsorption capacity of PB-immobilized membrane increased slightly at second recycle. Part of PB ligand-binding sites was hidden in a hydrophobic pocket on the surface of the membrane matrix. These caused steric hindrance of PB ligand–bilirubin interaction and reduced the contact between PB and bilirubin, which decreased the efficiency of bilirubin adsorption at first time. The surface structure of membrane was destroyed after the first adsorption test in alkaline condition (pH value is 11) so that the interactive sites of membrane were exposed completely.
Bilirubin adsorption comparison of all affinity membranes
Under identical condition, the bilirubin adsorption capacity of other affinity membrane was compared to this work and the result was showed in Table 3. In this work, the bilirubin adsorption capacity of PB-immobilized cellulose membrane reached 26 mg/g at pH 9.5, which was lower than that of CB-immobilized nylon membrane (30 mg/g). But the adsorption time of PB-immobilized cellulose membrane (3 h) was much lower than that of CB-immobilized nylon membrane (10 h). It means that PB-immobilized cellulose membrane has excellent adsorption performance. Moreover, 23% of total BSA amounts (11 g/L BSA solution) was adsorbed by CB-immobilized nylon membrane and BSA adsorption capacity of human serum albumin modified polytetrafluoroethylene membrane (40 g/L BSA solution) was 3.3 mg g−1. PB-immobilized cellulose membrane had no adsorption to BSA (10 g/L BSA solution) in this study, which confirmed the specific adsorption of PB-immobilized cellulose membrane to bilirubin and promising potential to application. This performance should contribute to the specific π-π interaction between PB and bilirubin.
Conclusion
In this work, the cellulose membrane was obtained by a phase inversion technique, and further grafted with Procion Blue H-5R molecules by nucleophilic reaction in alkaline condition. The amount of PB immobilized on cellulose membranes reached 230 mg/g membrane and bilirubin adsorption capacity reached 26 mg/g membrane. The bilirubin adsorption capacity of PB-immobilized membrane increased with initial bilirubin concentration increased. The adsorption isotherms indicated that the bilirubin adsorption process of PB-immobilized membrane fits the Langmuir isotherm well. The bilirubin adsorption capacity decreased rapidly with BSA concentration increased. High NaCl concentration and pH value played the negative influences on bilirubin adsorption. The bilirubin adsorption capacity of PB-immobilized membrane barely decreased after 3 recycles. Procion Blue H-5R could be expected as a good ligand for specific removal of bilirubin.
References
Altintas EB, Turkmen D, Karakoc V, Denizli A (2011) Efficient removal of bilirubin from human serum by monosize dye affinity beads. J Biomater Sci 22:957–971
Avramescu ME, Sager WF, Borneman Z, Wessling M (2004) Adsorptive membranes for bilirubin removal. J Chromatogr B 803:215–223
Bayramoglu G, Yalcin E, Arica MY (2005) Characterization of polyethylenimine grafted and Cibacron Blue F3GA immobilized poly (hydroxyethylmethacrylate-co-glycydylmethacrylate) membranes and application to bilirubin removal from human serum. Colloids Surf A 264:195–202
Chen J, Cheng G, Chai Y, Han W, Zong W, Chen J, Li C, Wang W, Ou L, Yu Y (2018) Preparation of nano-CaCO3/polystyrene nanocomposite beads for efficient bilirubin removal. Colloids Surf B 161:480–487
Dou Y-F, Zhang D-H, Liu H, Xia Y-P, Zhi G-Y (2018) Polyethyleneimine grafting and Cibacron Blue F3GA modifying poly (methylmethacrylate) magnetic microspheres for protein adsorption. J Chem Technol Biot 93:994–1002
Guo L, Zhang L, Zhang J, Zhou J, He Q, Zeng S, Cui X, Shi J (2009) Hollow mesoporous carbon spheres-an excellent bilirubin adsorbent. Chem Commun 40:6071–6073
Guo L, Zhang J, He Q, Zhang L, Zhao J, Zhu Z, Wu W, Zhang J, Shi J (2010) Preparation of millimetre-sized mesoporous carbon spheres as an effective bilirubin adsorbent and their blood compatibility. Chem Commun 46:7127–7129
Jiang X, Zhou D, Huang X, Zhao W, Zhao C (2017) Hexanediamine functionalized poly (glycidyl methacrylate-co-N-vinylpyrrolidone) particles for bilirubin removal. J Colloid Interface Sci 504:214–222
Liang-Schenkelberg J, Fieg G, Waluga T (2017) Molecular insight into affinity interaction between Cibacron Blue and proteins. Ind Eng Chem Res 56:9691–9697
Liu R-L, Ji W-J, He T, Zhang Z-Q, Zhang J, Dang F-Q (2014) Fabrication of nitrogen-doped hierarchically porous carbons through a hybrid dual-template route for CO2 capture and haemoperfusion. Carbon 76:84–95
Lu P, Hsieh Y-L (2009) Lipase bound cellulose nanofibrous membrane via Cibacron Blue F3GA affinity ligand. J Membr Sci 330:288–296
Lu P, Hsieh Y-L (2010) Layer-by-layer self-assembly of Cibacron Blue F3GA and lipase on ultra-fine cellulose fibrous membrane. J Membr Sci 348:21–27
Ma Z, Kotaki M, Ramakrishna S (2005) Electrospun cellulose nanofiber as affinity membrane. J Membr Sci 265:115–123
Ma C-F, Gao Q, Xia K-S, Huang Z-Y, Han B, Zhou C-G (2017a) Three-dimensionally porous graphene: a high-performance adsorbent for removal of albumin-bonded bilirubin. Colloids Surf B 149:146–153
Ma C-F, Gao Q, Zhou J, Chen Q-X, Han B, Xia K-S, Zhou C-G (2017b) Facile one-pot synthesis of magnetic nitrogen-doped porous carbon for high-performance bilirubin removal from BSA-rich solution. RSC Adv 7:2081–2091
Moosavi-Movahedi Z, Bahrami H, Zahedi M, Mahnam K, Chamani J, Safarian S, Saboury A, Moosavi-Movahedi A (2007) A theoretical elucidation of bilirubin interaction with HSA’s lysines: first electrostatic binding site in IIA subdomain. Biophys Chem 125:375–387
Ng I-S, Song C, Ooi CW, Tey BT, Lee Y-H, Chang Y-K (2019) Purification of lysozyme from chicken egg white using nanofiber membrane immobilized with Reactive Orange 4 dye. Int J Biol Macromol 134:458–468
Ostrow JD, Mukerjee P, Tiribelli C (1994) Structure and binding of unconjugated bilirubin: relevance for physiological and pathophysiological function. J Lipid Res 35:1715–1736
Peng Z, Yang Y, Luo J, Nie C, Ma L, Cheng C, Zhao CS (2016) Nanofibrous polymeric beads from aramid fibers for efficient bilirubin removal. Biomater Sci 4:1392–1401
Shi W, Cao H, Song C, Jiang H, Wang J, Jiang S, Tu J, Ge D (2010) Poly(pyrrole-3-caPBoxylic acid)-alumina composite membrane for affinity adsorption of bilirubin. J Membr Sci 353:151–158
Sochacka J (2015) Application of phenol red as a marker ligand for bilirubin binding site at subdomain IIA on human serum albumin. J Photochem Photobiol, B 151:89–99
Tamahkar E, Babaç C, Kutsal T, Pişkin E, Denizli A (2010) Bacterial cellulose nanofibers for albumin depletion from human serum. Process Biochem 45:1713–1719
Tao G, Zhang L, Hua Z, Chen Y, Guo L, Zhang J, Shu Z, Gao J, Chen H, Wu W, Liu Z, Shi J (2014) Highly efficient adsorbents based on hierarchically macro/mesoporous carbon monoliths with strong hydrophobicity. Carbon 66:547–559
Wang W, Zhang H, Zhang Z, Luo M, Wang Y, Liu Q, Chen Y, Li M, Wang D (2017) Amine-functionalized PVA-co-PE nanofibrous membrane as affinity membrane with high adsorption capacity for bilirubin. Colloids Surf B 150:271–278
Wang SS-S, Yang S-M, Hsin A, Chang Y-K (2018) Dye affinity nanofiber membrane for adsorption of lysozyme: preparation and performance evaluation. Food Technol Biotechnol 56:40–50
Wei H, Xu L, Ren J, Jia L (2012) Adsorption of bilirubin to magnetic multi-walled carbon nanotubes as a potential application in bound solute dialysis. Colloids Surf A 405:38–44
Xia B, Zhang G, Zhang F (2003) Bilirubin removal by Cibacron Blue F3GA attached nylon-based hydrophilic affinity membrane. J Membr Sci 226:9–20
Zhang L, Gu J (2006) Novel method for bilirubin removal from human plasma within modified polytetrafluoroethylene capillary. React Funct Polym 66:1106–1117
Zhu J, Yang J, Sun G (2011) Cibacron Blue F3GA functionalized poly (vinyl alcohol-co-ethylene) (PVA-co-PE) nanofibrous membranes as high efficient affinity adsorption materials. J Membr Sci 385–386:269–276
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, M., Sun, J. & Chen, L. Procion Blue H-5R functionalized cellulose membrane with specific removal of bilirubin. Cellulose 26, 8073–8085 (2019). https://doi.org/10.1007/s10570-019-02660-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02660-1