Abstract
The present study investigated chitosan nanoparticle (CS-TPP NPs) preparation by ionic gelation process with chitosan (CS) and tripolyphosphate (TPP). The structural characterization of nanoparticles was studied using Dynamic Light Scattering, Nuclear Magnetic Resonance, Fourier Transform Infrared (FTIR) and X-Ray Diffraction spectroscopies. FTIR spectra confirmed that the tripolyphosphoric groups of TPP linked with ammonium groups of CS in the prepared nanoparticles. CS-TPP NPs exhibited higher antioxidant activity and an interesting antimicrobial potential. In fact, there was no report available in literature on using CS nanoparticles for the surimi preservation. Further, data revealed that nanoparticle incorporation in fish surimi effectively inhibited thiobarbituric acid substances and conjugated dienes formation, and thereby, retarding lipid oxidation. Results showed that CS-TPP NPs were able to hinder fish surimi myoglobin oxidation with a significant improvement in the transformation of Heme iron. In addition, CS nanoparticles exhibited a distinguishable antimicrobial activity in the stored surimi during cold storage of 9 days. Thus, nanoparticles could be used as a natural ingredient to prevent lipid oxidation in surimi based food systems for the development of novel healthy fish products and addresses consumer demands for functional fish products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past two decades, the synthesis of nanoparticles is one of the expanding research areas in nanotechnology. With their novel properties, nanoparticles have been applied in several applications particularly medicine and other bio-related applications, such as drug delivery, photothermal therapy, wound healing and anticancer (Ruan et al. 2015; Kim et al. 2013). In addition, the nanoscale materials have been investigated for antimicrobial activity (Cioffi et al. 2005), as killing agents (Huang et al. 2003), antibiotic carriers (Gu et al. 2003) or for antitumor treatment (Bertrand et al. 2014).
Chitosan is a cationic polysaccharide composed of 2-acetamido-2-deoxy-β-D-glucan and 2-amino-2-deoxy-β-D-glucan units. This well-known polymer was mainly produced from chitin, present in cuticles of arthropods, the cephalopods endoskeletons and cell walls of fungi (Kaya et al. 2017). Chitosan polymer revealed very interesting physicochemical and biological properties, such as biodegradability, biocompatibility, bioactivity, high permeability, cost effectiveness and non-toxicity. Several studies have proposed CS as a biomaterial for pharmaceutics (Kim et al. 2008), tissue engineering (Li et al. 2005a, b), cosmetics (Kim et al. 2006), food preservation (Kanatt et al. 2008) and dietary (Anraku et al. 2009), due to its antibacterial and antifungal activities (Akmaz et al. 2013) as well as immune stimulatory properties (Meshkini et al. 2012).
Chitosan-based nanoparticles were produced and used as a suitable technological platform in different applications such as gene and drug delivery. Further, different methods were studied for chitosan-based nanoparticle preparation, such as emulsion-droplet coalescence (Tokumitsu et al.1999), emulsion solvent diffusion (Phromsopha and Baimark 2010), reverse micellization (Kafshgari et al. 2012), reverse emulsion (Divya and Jisha 2017), desolvation, or electrostatic complexation (Maciel et al. 2017). In fact, the ionic gelation appeared to be very attractive and reproducible method that produced nanometer sized particles with a narrow size distribution, as well as a highly positive surfaces charge. Among chemical products for nanomaterial preparation, TPP was used due to its physicochemical properties, bioactivity and environmentally friendly material and non-toxicity crosslinking-agent features, replacing glutaraldehyde or formaldehyde (Yang et al. 2010). The CS nanoparticles can be obtained by ionic gelation, when the positively charged amino groups of CS form electrostatic interactions with polyanions used as cross-linkers, such as TPP (Hosseini et al. 2015).
The CS-TPP NPs are preferably used in various applications owing to their biodegradability, biocompatibility, non-toxicity and cost effectiveness (Du et al. 2009; Arulmozhi et al. 2013). Chuah et al. (2011) and Alishahi et al. (2011) revealed that CS nanoparticles have been applied strongly in biomedical fields as agents of drug delivery, vaccine delivery and gene therapy. In addition, nanoparticles could enhance antioxidant properties and thermal stability of encapsulated drugs and natural polyphenol (Jung et al. 2015). In fact, the most studies published to date have been focused on the chemical characteristics, such as molecular weight, acetylation degree, pH, concentration, CS to crosslinker ratio, ionic strength and the factors (temperature, rate of stirring, type of stirring, rate of addition of crosslinker). However, the CS/TPP ratio effect on biological (antioxidant and antimicrobial) activities, as well as the functional properties are often underestimated or insufficiently described. Therefore, the present study focused primarily on CS/TPP ratio effects on the physicochemical and biological properties of nanoparticles in order to their uses as an alternative to chemical food additive.
Further, to the best of our knowledge, there are no studies have been focused in the literature on using CS-TPP NPs as a conservative agent of food products. Thus, in our study, CS-TPP NPs were used in the fish surimi formulation due to their higher antioxidant and antimicrobial properties. Therefore, the changes in pH, weight loss, lipid oxidation, textural analysis and microbial growth of fish surimi during 9 days of chilled storage at 4 °C as affected by CS and CS-TPP NPs addition were performed.
Materials and methods
Materials
Chitosan (deacetylation degree of 90% and mean molecular weight of 6120 g/mol) was extracted from crab shells as previously reported (Hajji et al. 2014). Acetic acid, TPP, thiobarbituric acid (TBA) and trichloroacetic acid (TCA) were purchased from Sigma Aldrich Chemical Co., Ltd.
CS-TPP NPs preparation
CS-TPP NPs were prepared by ionic gelation method by using CS and TPP. Briefly, CS (0.5%, w/v) was dissolved in acetic acid solution (2%, v/v). CS-TPP NPs were spontaneously formed by adding 10 ml of TPP solution (0.7 mg/ml) slowly drop wise into CS solution under vigorous stirring for 30 min at room temperature (25 ± 1 °C). In our study, the effects of CS/TPP mass ratio parameter (3/1, 4/1, 5/1 and 6/1) on CS-TPP NPs properties was investigated and nanoparticles were labeled CS-TPP NP3, CS-TPP NP4, CS-TPP NP5 and CS-TPP NP6, respectively. Subsequently, the prepared mixtures were ultrasonicated using an ultrasonic probe with diameter of 20 mm and 300 W high-intensity ultrasonic processor operating at 20 kHz for 5 min to reduce the agglomeration of nanoparticles. The resulted colloidal suspensions were centrifuged at 10,000 × g for 15 min and the pellets were re-suspended in ultrapure water. Nanoparticles were washed twice to remove un-reacted substances, freeze–dried and then stored at 4 ± 1 °C for further uses.
Characterizations of CS-TPP NPs
Nanoparticle recovery
The nanoparticle recovery was determined by comparing the initial weights of CS and TPP powders used for the nanoparticle preparation with the dry weights of nanoparticles suspension after centrifugation at 10,000 × g for 15 min and then freeze–dried. The recovery of nanoparticles was determined using the following equation:
Structural characterization
The particle size and size distribution of CS-TPP NPs were determined by dynamic light scattering (DLS) method using Zeta sizer Nano ZS (Malvern Instrument, UK) at room temperature (25 ± 1 °C) and a scattering angle of 90°. Distilled water was used as a reference for dispersing medium.
FTIR analysis were recorded in range of 4000–400 cm−1 using an FTIR spectrometer (Spectrum 2000, Perkin Elmer, USA). The structural characterization and crystallinity indices of CS and CS-TPP NPs were determined by using X-ray diffractometer (D8, Advance Bruker XRD diffractometer, Germany). The X-ray powder patterns were recorded using Ni-filtered Cu-Kα radiation of 1.54 Å, 50 kV and current of 100 mA. The samples were scanned from 2θ angles 5 to 80° at a scan speed of 0.5°/min. The crystallinity indices (Cr I) of nanocomposite films were determined using the following formula:
where: Ic and Ia are the intensities of crystalline peaks at 2θ = 20° and the amorphous diffraction at around 16°, respectively.
The characterization of CS and CS-TPP NPs by NMR solid-state 13C and 31P CP/MS spectroscopy was carried out using a Bruker W300 spectrometer with a frequency of 75.5 MHz, 50 ms acquisition time, 8 ms contact time and repetition time of 5 s.
Surface tension measurement and thermal properties of CS-TPP NPs
The surface tension of CS and CS-TPP NPs suspensions was measured according to the Du-Nouy methodology using a tensiometer TD1 (Lauda Königshofen, Germany). The thermal stability of CS-TPP NPs powders was studied using thermogravimetric analyzer (TGA Q500 High Resolution, TA Instruments), and operating under nitrogen flow. In fact, the TGA analysis was based on the mass change of samples as a function of temperature augmentation and a thermogram, showing the progressive change in mass in percentage as a function of temperature, is recorded. The CS and CS-TPP NPs samples were heated from 25 to 700 °C at a heating rate of 20 °C/min. The weight of nanoparticles samples, initially about 5 mg, was constantly measured with an accuracy of 0.01 mg.
The thermal properties of CS and CS-TPP NPs were further studied by using Differential Scanning Calorimeter (DSC, Modulated DSC Q20, TA Instruments) equipped with a liquid nitrogen cooling system. The DSC was used to estimate the melting and crystallization points, as well as the glass transition of the prepared nanoparticles. CS-TPP NPs samples were accurately weighed into sealed aluminum pans. An empty capsule serves as an inert reference and the apparatus was calibrated using indium. The thermal profile of samples was analyzed in a temperature range of 0–200 °C at a heating rate scan of 10 °C/min and under a nitrogen flow rate of 50 ml/min. Thermograms were then analyzed by using TA Universal V4.5A software.
Solubility of CS-TPP NPs
The solubility of CS-TPP NPs was determined according to the method of Fernandez-Kim (2004). CS and CS-TPP NPs powders (0.1 g) were placed into a pre-weighed centrifuge tube, and then dissolved in 10 ml acetic acid (1%) for 30 min at 25 ± 1 °C, under constant stirring. The solutions were then heated in a boiling water bath for 10 min. After cooling at room temperature (25 °C), centrifugation was carried out at 8000 × g for 10 min, and the supernatant was decanted. The pellet was dissolved in distilled water (25 ml) and then centrifuged at 8000 × g. The supernatant was discarded and undissolved pellet was dried at 50 ± 1 °C for 24 h. The solubility was determined as follow:
where W1 and W2 are the initial weight of (tube + sample) and the final weight of (tube + sample), respectively, and W0 is initial mass of tube.
Antioxidant activity of CS-TPP NPs
2,2-Diphenyl-1-picrylhydrazyl-(DPPH) free radical-scavenging activity
DPPH free radical-scavenging activity of CS-TPP NPs was determined according to the method described by Yen and Wu (1999). A volume of 500 µl of each sample at different concentrations was added to 375 µl of ethanol and 125 µl of DPPH (0.2 mM) dissolved in ethanol (99.50%) and mixed thoroughly. After that, the mixture was kept for 60 min in dark at room temperature (25 ± 1 °C) and the absorbance was measured at 517 nm. The lower absorbance of the reaction mixture indicated a higher free radical-scavenging activity. DPPH radical-scavenging activity was calculated as follow:
where Acontrol and Asample are the absorbances of control and sample reactions, respectively. BHA was used as a positive control. The analysis was carried out in triplicate.
Ferric reducing power assay
The ability of the prepared CS-TPP NPs to reduce iron (III) was determined as described by Yildirim et al. (2001). An aliquot of 0.5 ml of each nanoparticles sample was mixed with 1.25 ml of phosphate buffer (0.2 M, pH 6.6) and 1.25 ml of potassium ferricyanide (1%, w/v). The mixture was incubated at 50 ± 1 °C for 30 min and the reaction was stopped by addition of 1.25 ml of TCA (10%, w/v). Finally, 1.25 ml solution from the mixture was mixed with 1.25 ml of distilled water and 250 µl of ferric chloride (0.1%, w/v) solution. The mixture was incubated at room temperature (25 ± 1 °C) for 10 min and the absorbance was measured at 700 nm. BHA was used as a positive control.
β-carotene bleaching method
The capacity of CS-TPP NPs to prevent the bleaching of β-carotene was performed as described by Koleva et al. (2002). Thus, 0.5 mg of β-carotene was dissolved in 25 µl of linoleic acid, 200 µl of Tween-80 and 1 ml of chloroform. The mixture was completely evaporated under vacuum in a rotary evaporator at 50 °C. Thereafter, 100 ml of bi-distilled water was added, and the resulting solution mixture was vigorously stirred. A volume of 2.5 ml of the β-carotene/linoleic acid-emulsion was transferred to test tubes containing 0.5 ml of each sample. Following incubation for 1 h at 50 ± 1 °C, the absorbance was measured at 470 nm. A control reaction was consisted of 0.5 ml of distilled water instead of the sample. The same procedure was performed with BHA as a positive control. The β-carotene bleaching inhibition was calculated using the following equation:
where A0 and A ′0 are the absorbances, measured at time 0, of sample and control, respectively. At and A ′t are the absorbances, measured after 1 h, of sample and control, respectively. Tests were carried out in triplicate.
2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical-scavenging activity
The ABTS radical-scavenging activity of the prepared nanoparticles was determined as described by Wang et al. (2012) with slight modification. A volume of 1 ml of diluted ABTS solution was mixed with 1 ml of each CS-TPP NPs sample solution at various concentrations (1–5 mg/ml). The mixture was left at room temperature (25 ± 1 °C) in the dark. The absorbance was measured at 734 nm using a spectrophotometer against the corresponding control. The control was prepared in the same manner, except that distilled water was used instead of the sample. The ABTS scavenging activity of samples was calculated as follow:
where Acontrol and Asample are the absorbances of control and sample reactions, respectively.
Antimicrobial tests
The antibacterial activities of CS-TPP NPs solutions (10 mg/ml) were investigated against Bacillus cereus (ATCC 11778), Staphylococcus aureus (ATCC 25923), Micrococcus luteus (ATCC 4698), Salmonella enterica, Escherichia coli (ATCC 25922), Enterobacter, Salmonella typhimurium, Klebsiella pneumoniae (ATCC 13883) and Pseudomonas aeruginosa (ATCC 27853) bacteria using agar disc method. The antifungal activity was tested against Fusarium solani and Rhizoctonia solani strains. The culture suspension (100 μl) of each tested microorganism (106 CFU/ml for bacteria and 108 spores/ml for fungal strains) were spread over the Luria–Bertani agar and Potato Dextrose Agar medium, respectively. After that, 60 μl of each CS-TPP NPs solution was delivered into wells (5-mm depth, 5-mm diameter) previously cut in the agar. The antagonistic zones were detected after incubation at 37 ± 1 °C for 24 h for bacteria and 72 h at 30 ± 1 °C for fungal strains. Antimicrobial activity was evaluated by measuring the diameter of growth inhibition zones in millimeters (including well diameter of 5 mm).
Water and fat-binding capacities
The water and fat-binding capacities (WBC, FBC) of CS and CS-TPP NPs were determined as referred to Ocloo et al. (2011). Briefly, 0.5 g of sample was placed in a pre-weighed centrifuge tube and 10 ml of distilled water (or corn oil) were added. CS-TPP NPs were then suspended by means of a vortex mixer for 1 min, before left at ambient temperature (25 ± 1 °C) for 30 min, with intermittent shaking for 5 s every 10 min. After being centrifuged at 5000 × g for 25 min, the supernatants water or oil were decanted and the tube was weighed again. Tests were carried out in triplicate.WBC or FBC were calculated using the following equation:
Surimi preservation using CS-TPP NPs
Fish samples
Frigate grey mullet fishes with an average weight of 80–100 g were purchased caught from local supermarket in Sfax, Tunisia. The fish, off-loaded approximately 12 h after capture, was placed in ice with a fish/ice ratio of 1:2 (w/w) and transported to the laboratory within 20 min. Fish were immediately washed, gutted, filleted, skinned and the whole muscles were collected. To prepare fish mince, fish fillets were minced to uniformity using a meat grinder. The muscles were kept on ice during preparation.
Surimi preparation
To prepare surimi by conventional washing process, fish mince was washed with cold water (4 ± 1 °C) using a water/mince ratio of 3:1 (v/w). The mixture was stirred gently for 10 min in a cold room (4 ± 1 °C) and the washed mince was filtered with a layer of nylon screen. Washing was performed three times. Finally, the washed mince was pressed and was referred to as ‘surimi’. The surimi was subdivided on three groups as follow: Control lot: Surimi sample without any supplementation; Chitosan lot (CS): Surimi sample supplemented with CS (1%, g/g of surimi) and CS-TPP NPs lot: Surimi sample supplemented with CS-TPP NPs (1%, g/g of surimi). The surimi samples were cooked at 90 °C for 20 min, cooled at room temperature (25 ± 1 °C) and then stored at 4 ± 1 °C until uses. Groups were sampled at 1, 3, 6 and 9 days of storage for physicochemical characteristics and microbial analyses.
Moisture, weight loss and pH measurements of surimi
Moisture content in surimi samples was determined in triplicate by the oven drying method at 105 ± 1 °C until constant weight. The pH values of surimi from each treatment were measured. Thus, 10 g of the sample was homogenized with 50 ml of distilled water for 2 min using an Ultra-Turrax apparatus (IKA T18 Basic, Germany). The sample was transferred into a beaker and pH was determined using a combined glass electrode pH meter (Metrohm AG pH lab system, France) by directly immersing the electrode into the homogenates.
Weight loss (WL) of surimi samples was monitored during the storage using an analytical balance by weight determination at 1, 3, 6 and 9 days (Wi), as compared to their initial weight (W0) and expressed as follow:
were W0 and Wi are the initial and final weights of samples, respectively.
Myoglobin content determination
The myoglobin content in surimi was determined by direct spectrophotometry measurement as described by Benjakul and Bauer (2001). An aliquot of 2 g of each sample was weighed into a 50 ml polypropylene centrifuge tube and 20 ml of cold phosphate buffer (40 mM, pH 6.8) was added. The mixture was homogenized at 13,500 rpm for 10 s, followed by centrifugation at 3000 × g for 30 min at 4 ± 1 °C. The supernatant was filtered with Whatman No. 1 filter paper. The myoglobin content was determined at 555 nm, and calculated from the millimolar extinction coefficient of 7.6 and molecular weight of 16,400 (Gomez-Basauri and Regenstein 1992). The myoglobin content was expressed as mg/g of sample.
Metmyoglobin determination
The oxidation of myoglobin of surimi samples from different groups was determined spectrophotometrically. Metmyoglobin determination was performed as described by Hansen and Sereika (1969). The ratio of A630/A525 was calculated. In fact, a high A630/A525 ratio indicates a high relative proportion of metmyoglobin.
Lipid peroxidation assessment
Oxidative rancidity of surimi samples was measured by a TBARS assay of malondialdehyde (MDA). The absorbance was measured at 535 nm using an UV–Vis spectrophotometer. The TBARS values were calculated using molar absorption of MDA (156,000 M/cm) at 535 nm. The TBARS values were reported as mean values (± standard deviation) of at least three replicates and the mean values were expressed as µg of MDA per g of surimi sample.
Lipid oxidation was likewise evaluated by the conjugated dienes content and measured at 233 nm (Esterbauer et al. 1982).
Texture profile analysis
Texture profile analysis of surimi was performed using a TA-XT2i texture analyzer (Stable Micro Systems, Godalming, Surrey, UK). Samples were subjected to two-cycle compression at 50% compression using the texture analyzer with a 1-cm TPA compression plate attachment moving at a speed of 10 mm/min. From the resulting force–time curves, hardness, cohesiveness ratio, springiness, chewiness and adhesiveness were evaluated using the texture analyzer. Hardness was measured with a probe during the first compression. Cohesiveness ratio was the measure of the area of work during the second compression divided by the area of work during the first compression. Springiness was calculated as the force at maximum compression during the second compression cycle. Chewiness was determined as the product of gumminess and springiness by measuring the energy required to chew a solid sample to a steady state of swallowing. Finally, the adhesiveness was defined as the negative force area under the curve obtained between cycles.
Microbiological analysis
In order to determine the bacteriological counts in the preserved surimi, 1 g portion of sample from each group was aseptically transferred into sterile tube and then homogenized with 9 ml of sterile saline solution (0.9%, w/v). From this solution, tenfold serial dilution of surimi homogenates were prepared and used for bacterial and fungal enumeration. Total psychrotrophilic and mesophilic bacteria were determined by the pour plate technique using plate count agar and incubated for 24–72 h at 4 ± 1 °C and 37 ± 1 °C, respectively. Yeast and molds counts were performed using surface seeding technique with potato dextrose agar for 3–5 days at 30 ± 1 °C. All microbial counts were expressed as logarithms of colony-forming units per gram of surimi (log CFU/g).
Statistical data analysis
All experiments were carried out in triplicate, and the average values with standard deviation errors were reported. Mean separation and significance were analyzed via the SPSS software package ver. 13.0 professional edition (SPSS, Inc., Chicago, IL, USA),using ANOVA analysis based on Duncan’s multiple range test to estimate the significance among the main effects at the 5% probability level.
Results and discussion
Synthesis and characterization of CS-TPP NPs
CS/TPP mass ratio is critical to control the particle size and size distribution. Different CS/TPP mass ratios (3/1, 4/1, 5/1 and 6/1) were prepared using a constant CS concentration (0.5 mg/ml) and the average hydrodynamic particles size of CS-TPP NPs was analyzed by DLS (Fig. 1A). A strong dependence of the particle size and size distribution could be observed according to the ratio CS/TPP (Table 1). The highest particle size for the prepared CS-TPP NPs was attained for CS/TPP ratio of 3/1 (321.5 nm) (CS-TPP NP3) and steadily decreased as the ratio CS/TPP is going up, reaching 121.6 nm for CS-TPP NP6. This effect could be explained by the increase in the crosslinking density as the ratio CS-TPP is getting higher. Indeed, with higher concentration in CS in the solution, more positive charges will be available to interact ionically with the negative charges of multivalent TPP, giving rise to a dense crosslinked network. The higher crosslinking density to more compact will be the network that will segregate in tiny nanoparticles under the effect of sonication.
Chitosan nanoparticles were formed spontaneously due to the interaction between the negatively charged groups of TPP and the positively charged amino groups of CS, as reported earlier (Calvo et al. 1997). Further, TPP addition on CS solution leads to ionic gelation and thus nanoparticle formation. Dudhani and Kosaraju (2010) reported that size distribution of CS-TPP NPs was ranged from 20 to 600 nm. However, Nguyen et al. (2017) showed that the mean size of CS nanoparticles was in range of 166–1230 nm, depending on the molecular weight of CS and size of the spray dryer nozzles. In addition, results showed that the prepared CS-TPP NPs were characterized by a high recovery percent in the range of 100–201% (Table 1), depending on the CS/TPP ratio used for the CS-TPP NPs preparation.
It was shown that CS had a surface activity at concentration of 0.5 mg/ml about 46.1 mN/m, which is in agreement with data reported by Calero et al. (2010). The surface activity was explained by the formation of CS monolayer at the air–water interface, hiding water molecules from the surface.
The surface (water–air) tension of CS-TPP NPs solutions has been studied. Results showed an increase in the interfacial tension for the NPs with the increase of CS/TPP ratio mass. This effect is likely due to the consumption of CS from the solution, leaving a small fraction of CS available at the air–water interface. This result also is indicative of a lack of surface activity of CS-TPP NPs, presumably due the impossibility of adsorb at water–air interface.
FTIR analysis
FTIR spectroscopy was used in order to confirm the linkage between phosphoric and ammonium ions of TPP and CS in the prepared nanoparticles (Fig. 1B). In the CS spectra, the characteristic peaks were observed at 3365 cm−1 (–OH and –NH2 stretching), 2870 cm−1(–CH stretching), 1660 cm−1 (amide I) and 1075 cm−1 (C–O–C asymmetric stretching). The sharp peaks at 1371 cm−1 and 1427 cm−1 can be assigned to the –CH3 symmetrical stretching band (Dudhani and Kosaraju 2010). For the band at 1060 cm−1, it was assigned to the second –OH group of CS polymer. For FTIR spectrum of TPP powder, two intense absorption peaks observed at 1210 cm−1 and 860 cm−1 were assigned to P=O stretching and P–O along with P–O–P (Rodrigues et al. 2012).
By comparing FTIR spectra of CS-TPP NPs and CS, several changes were observed. The bands corresponding to the O–H and N–H stretching vibrations (3334 and 2884 cm−1, respectively) were broader indicating the involvement of -OH and -NH groups in the crosslinking process, presumably through hydrogen bonding within CS-TPP network. In addition, the C=O and –NH2 vibrations bands (1660 and 1583 cm−1) shifted to lower wavenumber (1635 and 1547 cm−1), which clearly confirms the electrostatic interaction between NH3+ groups of CS and the polyphosphoric groups of TPP (Dudhani and Kosaraju 2010; Jingou et al. 2011). Moreover, the band observed at 1251 cm−1 strongly weakened in intensity or even vanished with emergence of a new peak around 1218 cm−1 (C–O–C stretching). The bands at 1203 and 895 cm−1 in the CS-TPP NPs spectra were attributed respectively to P=O stretching vibration and P–O–P asymmetric stretching vibration, confirming the presence of P–O–P linkage within CS-TPP NPs. The bending vibration in C–H groups of acetyl (1371 cm−1) shifted to 1380 cm−1 for the CS-TPP NPs. At 1070 and 690 cm−1, the peaks were assigned to stretching of C–O–C and stretching of the pyranoside ring (Hebeish et al. 2013). The shifts and the changes in the vibration frequencies in this particular region is due to electrostatic interaction between amino groups of CS and polyphosphoric groups of TPP (de Pinho Neves et al. 2014).
X-ray diffraction
The XRD analysis was carried out to determine the crystallinity of the materials. Pure CS shows diffraction peaks at 2θ = 10.05° and 19.86° (Fig. 1C) corresponding to the respective equatorial (020) and (110) of the microcrystalline reflections of CS (Jingou et al. 2011). After ionic cross-linking with TPP, the diffraction peaks were observed at 12.85, 18.83 and 27.26°, with substantially lower intensity, indicating that CS-TPP NPs are more amorphous than CS polymer, with almost no ordered structure (Jingou et al. 2011). This effect is expected if we consider that the crosslinking reaction reduces the possibility long range order within the CS-TPP network (Rokhade et al. 2006). The crystallinity indices of CS and CS-TPP NPs were 51.18% and 35.03%, respectively. Similar results were reported by León et al. (2017) for CS-TPP NPs dried with supercritical CO2.
13C and 31P NMR characterization
13C CP/MAS NMR spectra of CS and CS-TPP NPs are depicted in Fig. 2A. CS spectrum was characterized by the presence of five bands at 57.85, 60.08, 75.63, 83.31 and 105.31 ppm, that correspond to the pyranose rings carbon atoms (C2, C6, C3/C5, C4 and C1; respectively). Additionally, the methyl and carbonyl carbons of the acetyl group of N-acetyl-glucosamine units in CS (at 21 and 175 ppm, respectively) were reduced due to the deacetylation process. As compared to CS, CS-TPP NPs spectra showed similar peaks but broader and with lower resolution, which could be related to the layout of CS chains and TPP molecules forming an amorphous structure of CS-TPP NPs.
The 31P NMR spectra of CS-TPP NPs, as compared to TPP powder, were shown in Fig. 2B. As observed, the NMR spectra of the commercial TPP powder, used in the nanoparticle preparation, showed several peaks in the region between − 7 and 6 ppm, indicating that its powder represents a mixture of two crystalline phase polymorphs of Na5P3O10 and Na5P3O106H2O. Further, the 31P spectra of CS-TPP NPs showed a broad peak, characteristic of amorphous materials, suggesting the amorphous structure of phosphate groups in the prepared NPs. At − 6.79 ppm, it was observed a broad and asymmetric peak, which could be attributed to the terminal phosphate units in the amorphous TPP, and a small peak at − 19.28 ppm, characteristic of phosphate linked by two phosphate units (PO4/2)3+, suggesting the presence of interaction between CS and TPP in the CS-TPP NPs, that was achieved by the pyrophosphate units and few interactions by TPP (Vasconcelos et al. 2008). These results are in line with those cited by Barbi et al. (2015), who reported the same peaks and shifting to − 4.2 and − 17.3 ppm.
Thermal gravimetric analysis
TGA is a useful analytical analysis to determinate the weight loss of the prepared CS-TPP NPs as a function of temperature or time. The decomposition Temperature (Td) is the temperature corresponding to the maximum slopes of each weight change step. The Td is obviously seen as a peak when the rate of mass loss with respect to temperature in Derivative Thermo Gravimetry (DTG) thermograms. The TGA curves profiles revealed the decrease in weight of CS and CS-TPP NPs with increasing in the temperature from 30 to 600 °C (Fig. 3A). The CS is characterized by two major phases. The initial weight loss of about 6% around 100 °C in neat CS is due to moisture evaporation (Table 2). In CS-TPP NPs, the second weight loss of about 10% around 150 °C is observed which is due to removal of water entrapped within the CS-TPP network (dehydration). The shift of water evaporation to higher temperature is presumably due to the strong interaction of water molecules with CS-TPP network, presumably through strong hydrogen bonding. A third weight loss is observed around 300 °C is due to the thermal degradation of the D-glucosamine units of CS polymer. The residual mass of CS was at about 17.50% at 600 °C, corresponding to ash content composed of mineral residue of CS-TPP.
The thermal decomposition Td of CS-TPP NPs clearly observed in Fig. 3B was ranged from 294 to 298 °C (Table 2), which was lower than that of CS polymer (329.47%). Consequently, it can be deduced that CS and CS-TPP NPs were decomposed at a higher temperature, reflecting their improved thermal stability. Moreover, the total weight loss of CS-TPP NPs was nearly 37.51, 45.51, 42.45 and 32.48% for CS-TPP NPs 3, 4, 5 and 6, respectively. Thus, it could be concluded that thermal stability was correlated with the composition of CS-TPP NPs and especially the CS/TPP mass ratio. In fact, the use of TPP for the CS-TPP NPs preparation induced a modification in the crystalline nature of CS polymer, thus making the two molecules more stable on heating, which have also reported a good stability of CS-NPs.
The degradation profile of CS-NPs seems to be different compared to CS, but stable than CS which proves that the system is amorphous nature. When compared with CS-TPP NPs, the residual mass is higher than CS (17%). This tells that the CS not only has intramolecular hydrogen bonding but also intermolecular interactions with TPP during crosslinking. It confirms that the prepared chitosan nanoparticles have high thermal stability than pure CS polymer.
Differential scanning calorimetry
DSC analysis was used to confirm the crystal transformation of the CS-TPP NPs as compared to CS polymer. As clearly indicated in the DSC thermogram, CS-TPP NPs, a broad endothermic peak was observed in 90–100 °C range (Fig. 3C). However, CS showed an endothermic low peak at 100 °C that represents the melting temperature of CS (Piyakulawat et al. 2007). For the glass transition temperature (Tg) of CS and CS-TPP NPs was found to be 173.50 °C and 148–164 °C range, respectively, thus suggesting the thermal stability of the polymer (Table 2). This confirms that the thermal stability of CS was improved when it is crosslinked with TPP. It could be deduced from these results that the CS with blocked amino groups (for CS-TPP NPs) is thermally less stable than CS with free amino groups.
Solubility assessment
One of the key properties of CS and CS-TPP NPs is their solubility. As shown in Table 3, all CS-TPP NPs exhibited a higher solubility ranged from 82.07 to 86.32%. However, CS revealed the highest solubility (95.56%) in 1% acetic acid. Such aqueous and organic solvents solubility could be attributed to the crystalline structure of CS and CS-TPP NPs. Hamdi et al. (2017) reported that CS extracted from blue crab shells was completely soluble in 1% acetic acid.
Water and fat binding capacities
The WBC and FBC of CS and CS-TPP NPs were evaluated for their possible uses as functional ingredients in foods. As shown in Table 3, CS-TPP NPs improved both WBC and FBC since values were significantly higher than those of CS (24.76 and 14.49 g/g of sample), respectively. In addition, results demonstrated that there is a correlation between WBC, FBC and the CS/TPP ratio used for the NPs preparation. Hence, among the prepared NPs, CS-TPP NP6, with CS/TPP ratio of 6/1 and the lowest NPs size (121.6 nm) showed the best ability to bind water (33.18 g/g of sample), while CS-TPP NP5 (153.90 nm) presented the highest FBC (39.07 g/g of sample). In fact, the difference in the water and fat binding capacities could be attributed to the difference in the particles size.
Antioxidant abilities of CS-TPPNPs
To further confirm the antioxidant efficiency of the prepared CS-TPP NPs, four tests were performed such as DPPH radical scavenging activity, ferric reducing power, β-carotene bleaching-inhibition capacity and ABTS radical scavenging activity.
DPPH radical-scavenging ability
DPPH has an hydrogen free radical and displayed a distinctive absorption at 517 nm. The antioxidants can quench DPPH free radical and convert it into a colorless product, resulting in a decrease in absorbance. In the present study, DPPH radical scavenging capacity of CS-TPP NPs was investigated at different concentrations (1–5 mg/ml), as compared to the CS powder. As shown in Fig. 4A, CS-TPP NPs exhibited notable antioxidant activity against DPPH, in a dose-dependent manner, but still lower than BHA at the same concentration. Further, at 5 mg/ml, CS-TP NP4 exhibited the highest antioxidant activity (74.7%), which is comparable to those obtained for CS at the same concentration (76%).
The half-maximal inhibitory concentration (IC50) values, determined via the regression equation, for the prepared CS-TPP NPs were in the range of 1.7–3.2 mg/ml, against 2.4 mg/ml for pure CS. Therefore, these findings suggest that the amine groups in the CS-TPP NPs could react with free radicals to convert them to more stable molecules and terminate the radical chain reaction by donating protons. Hosseini et al. (2018) reported that fish peptide fraction encapsulated in CS-TPP NPs were characterized by a higher DPPH radical scavenging activity of 75.35% at 5 mg/ml, which is similar to the results of Chi et al. (2015).
β-carotene bleaching-inhibition
The β-carotene–linoleic bleaching inhibition assay is commonly used for the evaluation of the antioxidant activity. In this system founded on oil–water emulsion, in the absence of antioxidants, β-carotene undergoes fast bleaching, which generates free radicals creating peroxyl radicals. A free-radical scavenger can inhibit bleaching of β-carotene by neutralizing the linoleate-free radical as well as other free radicals formed during the reaction.
The ability of CS-TPP NPs to protect β-carotene against bleaching as compared to CS was tested. All CS-TPP NPs were able to catch the linoleic hydroperoxyl radicals in a dose-dependent manner (Fig. 4B). In fact, at 5 mg/ml, CS-TPP NP5and CS-TPP NP6 displayed an important antioxidant activity estimated at about 64% and 71%, respectively, while at the same concentration, the antioxidant activity of CS was about 70%. It can be concluded that CS-TPP NPs could inhibit the β-carotene-bleaching by the neutralization of the linoleate-free radical, as well as others free radicals formed during the reaction. Thus, CS NPs could be used as an antioxidant in food industries.
ABTS radical-scavenging activity
ABTS radical cations decolorization assay is a widely used method for the assessment of the antioxidant activity of various substances. As observed in Fig. 4C, the antioxidant activity of CS and CS-TPP NPs increased with increasing the concentration of CS NPs. The IC50 value of any antioxidant refers to the minimum amount of the substances that is required to scavenge 50% of the radicals generated through the in vitro system. The lowest IC50 value was obtained for CS-TPP NP6 (2.3 mg/ml), which was comparable to CS powder (2.1 mg/ml). Nallamuthu et al. (2015) reported a lower IC50 value of chlorogenic acid loaded with CS-TPP NPs (92 ± 5 μg/ml).
Reducing power assay
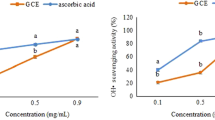
The reducing power ability of the prepared CS-TPP NPs is evaluated at different concentrations ranged from 1 to 5 mg/ml. The results showed a relatively low reducing effect (OD700 nm = 0.4–0.56 at 5 mg/ml) (Fig. 4D), in spite of BHA used as reference at the same concentration. These findings were additionally observed for CS (OD700 nm = 0.46 at 5 mg/ml). Consequently, the prepared CS-TPP NPs and CS were not effectives in the reduction of Fe3+ to Fe2+ by donating electron.
Antimicrobial activity
The damage of foods during the preservation is caused by free radicals, besides the contamination by microbes, which affects the quality of foods and could cause serious health problems to the consumers. The antibacterial activity of the prepared CS-TPP NPs at 10 mg/ml was assessed against two fungal strains and three Gram positive and six Gram negative bacterial strains. It can be concluded that the antibacterial activity depends on the particle size values of CS-TPP NPs (Table 4). In fact, nanoparticles characterized by low particle size values showed the highest bacterial growth inhibition. Among the tested samples, CS-TPP NP6, with lowest nanoparticles size (121.60 nm), showed the highest antimicrobial activity against B. cereus, M. luteus, S. enterica and Enterobacter with diameters of inhibition zone in the range of 18–23 mm, which was found to be higher than those of CS. However, TPP powder did not show any antibacterial effect on the different bacteria strains.
In line with Ali et al. (2010), CS-NPs prepared by ionic gelation characterized by a lower particle size (about 100 nm) showed an important antimicrobial activity against S. aureus bacteria than that of CS. The authors explained this behavior by a higher specific area of small particles, and by the fact that at pH 5.5–6.0, the amount of ammonium groups NH3+ involved in the nanoparticles formation is lower. It would result in a higher quantity of ammonium groups available for the interactions with the bacterial membranes, which increase the antimicrobial efficiency of NPs. Furthermore, Du et al. (2009) showed that CS-NPs exhibited MIC and MBC values against E. coli, Salmonella choleraesuis, Staphylococcus aureus about 4 times lower compared to CS. However, Qi et al. (2004) estimated the MBC value of CS-NPs 64 times inferior to CS solutions against E. coli K88.
The antifungal activity of CS-TPP NPs at 10 mg/ml was evaluated against two pathogenic fungi species (F. solani and R. solani) by determining the diameter of zone inhibition. As displayed in Table 4, all CS-TPP NPs have high inhibition rates against the tested fungi with inhibition zones between 16 and 19 mm. Interestingly, CS-TPP NPs exhibited a higher antifungal activity against F. solani and R. solani than CS (15 and 14 mm, respectively) at the same concentration. Results were in accordance with previous studies where the chitosan nanoparticles exhibited higher antimicrobial activity and inhibited 84.72% of Rhizoctonia solani, 76.72% of Collectotrichum acutatum and 32.16% of Phytophthora infestans at 50 mg/ml and 63.88% of Fusarium oxysporum at 40 mg/ml (Divya et al. 2018). The microscopic examination of fungal cultures showed that chitosan nanoparticles disrupt the cell membrane permeability and cause a morphological and molecular alteration of fungal cells. In fact, the exact mechanism of microbial inhibition by CS and CS-TPP NPs still debatable and might be attributed to the amino groups of CS, which promoted the structural disruption of bacterial and fungal cells. In addition, the antimicrobial properties of CS-NPs were also attributed to the small and compact particle size of nanoparticles as well as high surface charge (Qi et al. 2004).
Surimi preservation
Physicochemical properties of surimi
As shown in Fig. 5A, the initial pH values of surimi ranged from 6.71 to 6.84 for the three groups (Control, CS and CS-TPP NPs). The pH value of control group showed a decreasing trend during 9 days of storage (pH 6.25), as compared to the CS and CS-TPP NPs groups (pH 6.48 and 6.5). The decrease in pH values could be attributed to the precipitation of alkaline calcium, magnesium and sodium phosphates of the stored surimi during the 9 days. It can be concluded that CS and CS-TPP NPs were effective in maintaining the pH values and extending the surimi storage.
Determination of pH (A), weight loss (B), moisture content (C), myoglobin (D) and metmyoglobin (E) contents in fish surimi samples stored at 4 °C for 9 days. Values with different letters (a–c) within each parameter in the same day differ significantly (p < 0.05). Values with different letters (A–D) in the same treatment differ significantly (p < 0.05)
Furthermore, CS-TPP NPs addition in fish surimi formulation prevented the weight loss, increasing therefore the shelf life of the stored surimi (Fig. 5B). In fact, the weight loss of surimi was at about 10.8 and 6.4% for CS and CS-TPP NPs formulations after 9th day, respectively, compared to that of the control group (19.67%). CS and especially CS-TPP NPs were able to prevent the weight loss by increasing the resistance to water vapor transmission of fish surimi during the storage. Previous studies reported that the dense structure of CS makes it a very effective gas barrier (Plainsirichai et al. 2014).
During first day, the process of rehydrating the surimi was done properly, as the moisture content of CS and CS-TPP NPs formulations was similar (68–72% range), which was deal for commercial surimi production (Fig. 5C). Dey et al. (2013) prepared croaker fish surimi and reported a similar result. During the storage, it was observed a remarkable diminution of surimi moisture for the control group (50.9%) on 9th day. However, for the surimi incorporated with CS and CS-TPP NPs, there is no difference in the moisture percents (60.2 and 63.1%). Both CS and CS-TPP NPs additives could be effective in maintaining the moisture quantity of surimi during 9 days of cold storage. These results were in line with the weight loss of surimi during the storage.
Metmyoglobin and myoglobin contents
Metmyoglobin and myoglobin are two main pigments that constitute red color in muscle of fish. In fact, myoglobin concentration generally depends on species, sex and age of animals and the nature of nutrition, oxygen availability and muscle type, as well as the treatment of the meat (Postnikova et al. 1999). For the myoglobin content of fish surimi, there is no difference (3.18, 3.02 and 3.33 mg/g) for control, and the treatment with CS and CS-TPP NPs in the first day of storage (Fig. 5D). After 6 days of the cold storage, it was observed a remarkable decrease in the myoglobin content for the control group (2.39 mg/g), as compared to the other treatments (2.68 mg/g for CS and 2.7 mg/g for CS-TPP NPs groups). In fact, previous study reported that the prolonged cold storage reduced the myoglobin contents in sardine and mackerel muscle, which could be attributed to the insolubility of this pigment caused by the myoglobin oxidation to form the metmyoglobin (Chaijan et al. 2005). These changes were due to the intrinsic and extrinsic factors that determinate the metmyoglobin formation. The denaturation and/or oxidation of myoglobin could induce the myoglobin pigment binding with the structural muscle proteins (Ochiai et al. 2001; Chen 2002). Furthermore, Chaijan et al. (2004) showed that the myoglobin contents were higher in the dark muscle than in ordinary muscle of both sardine and mackerel fish. The myoglobin content of frigate mackerel surimi was 1.61 and 2.66 times higher than those of Indian mackerel surimi and short-bodied mackerel surimi, respectively (Chaijan et al. 2010).
The effect of CS and CS-TPP NPs incorporation on the surimi metmyoglobin content, during the 9 days of conservation at 4 °C was further investigated. As shown in Fig. 5E, data clearly revealed that there are no significant differences in the metmyoglobin amount (0.41–0.49) at the first day of storage. However, from the 3rd day of cold storage, it was observed a remarkable increase in the metmyoglobin content for the control group (0.62). For surimi supplemented with CS and CS-TPP NPs, a lower oxidation values were obtained with metmyoglobin values of 0.55 and 0.52, respectively. Interestingly, CS and CS-TPP NPs were found to be significantly more efficient in the stabilization and protection of myoglobin pigment than the control formulation. Results were in line with other study, which reported that the total pigments in sardine and mackerel muscles gradually decreased as the storage time increased and suggested that the presence of frigate mackerel and Indian mackerel surimi showed a detrimental effect on color of the surimi blend (Chaijan et al. 2006).
During the cold storage, an exponential increase of about 1.23 in the metmyoglobin content for the surimi without any additive was observed after the 9th day of storage. However, the CS and CS-TPP NPs addition induced a lower increase in the myoglobin oxidation (0.73 and 0.69). Chaijan et al. (2010) showed relative metmyoglobin contents of 0.65 and 0.44 for frigate mackerel and Indian mackerel surimi, respectively. Lee et al. (2003) reported that the metmyoglobin formation is positively correlated to the lipid oxidation. During the storage, discoloration of tuna was attributed to the formation of metmyoglobin pigment (Haard 1992).
Lipid oxidation of fish surimi
The MDA, the main secondary product of lipid oxidation, is widely studied as a marker of oxidative stress and lipid peroxidation indices were measured using the TBARS assay. The TBARS values are crucial indicators of the oxidative stability for seafood and derived food products. In fact, the high TBARS values of food products have been correlated with higher rancidity and lower consumer acceptance (Ke et al. 1984). The surimi products are highly susceptible to lipid oxidation, which can lead to flavor and color deterioration.
In the present study, the effect of CS and CS-TPP NPs incorporation on lipids oxidation in the surimi samples was investigated during 9 days of storage time at 4 °C. The MDA levels determination for the different groups of surimi, depicted in Fig. 6A, show that, independently of the formulation used, the MDA values increased significantly (p > 0.05) during the cold storage, but varied among the treatments. It was observed that the control group of surimi was the most susceptible to lipid peroxidation with significantly higher MDA values that increase significantly, as a function of storage time, as compared to the other treatments (CS and CS-TPP NPs) in all storage days. Interestingly, CS and especially CS-TPP NPs were found to exhibit an inhibitory role against lipid oxidation of surimi during the storage. After 9 days of storage time, control sample showed the highest MDA content (1.42 µg/g of surimi), while the surimi enriched with CS and CS-TPP NPs exhibited a lower MDA amounts of 0.52 and 0.45 µg/g of surimi, respectively.
Effects of CS and CS-TPP NPs incorporation on lipid peroxidation (A) and conjugated dienes (B) of fish surimi samples during the cold storage. Values with different letters (a–c) within each parameter in the same day differ significantly (p < 0.05). Values with different letters (A–D) in the same treatment differ significantly (p < 0.05)
Ke et al. (1984) proposed that MDA values for seafood products below 0.58 mg/kg are perceived as not rancid, 0.58–1.51 mg/kg slightly rancid, but acceptable, and above 1.51 mg/kg rancid. Using these ranges, surimi samples enriched with CS and CS-TPP NPs would be perceived as slightly rancid, but acceptable for the human consumption. Darmadji and Izumimoto (1994) reported that the addition of CS (1%) resulted in a 70% reduction of the TBARS values in beef after storage at 4 °C for 3 days.
The lipid peroxidation of the surimi products was equally monitored by the determination of conjugated dienes, which was recognized as the primary oxidation product. As shown in Fig. 6B, the conjugated dienes amounts increased with the storage time for all surimi groups, indicating the generation of hydroperoxides substances. For the surimi supplemented with CS and CS-TPP NPs, it was observed an increase in conjugated dienes from OD233 nm of 0.24 and 0.28 at 1st day to OD233 of 0.56 and 0.45 after 9 days of storage at 4 °C, respectively. However, for the control group, it was observed higher level of conjugated dienes after 9 days of storage time (OD233 nm = 0.7). Therefore, the incorporation of CS and CS-TPP NPs into surimi products delayed the oxidation phenomenon, as compared to the control group. These results suggest that both CS-TPP NPs probably contain bioactive agents responsible of the surimi protection against lipid peroxidation and able to delay the process of food oxidative degradation. In fact, Shahidi et al. (1999) reported that CS could chelate the free ions, which are released from hemoproteins during heat processing or storage, and thus inhibit the lipid peroxidation of products.
Texture profile analysis
Five factors are determined using texture profile analysis (TPA) of fish surimi at the first day and at the end of storage including hardness, springiness, cohesiveness, gumminess and chewiness. Table 5 shows the effects of CS and CS-TPP NPs addition on the texture features of fish surimi during storage at 4 °C. Results showed that the texture of samples was not deteriorated during the storage time. However, textural scores for control samples were significantly lower than those of the treated samples. At the first day, there is no significant difference for cohesiveness of the control and surimi enriched with CS and CS-TPP NPs groups. During the cold storage, the cohesiveness of the different surimi samples was reduced. In fact, the cohesiveness is a measure of the capacity of samples to maintain the interactions and the internal bonds in surimi after the first compression cycle of the TPA, thus the cohesiveness value affects the hardness value of surimi. At the 9th day, CS and CS-TPP NPs addition gave the highest cohesiveness value than the control groups. The improvement of surimi cohesiveness by the CS powder has been as well reported by Rajalekshmi and Mathew (2007) for the threadfin bream surimi during the frozen storage.
The surimi samples supplemented with CS revealed higher chewiness (24.07 N.mm) (p < 0.05) than CS-TPP NPs (16.39 N.mm) and the control (11.54 N.mm) groups at the first day of storage (Table 5). During the storage, the chewiness of the fish surimi was significantly reduced. However, the CS-TPP NPs addition improved the chewiness of the samples and was found to be the more effective treatment of surimi during the cold storage.
As observed in Table 5, the hardness values exhibited a significant (p < 0.05) decrease tendency with surimi conservation time. Colmenero et al. (1996) reported that protein contents were responsible for the hardness and were strongly influenced by protein concentration in processed muscle foods. At the end of the storage time, the highest hardness value was obtained for surimi enriched with CS (11.01 N) and CS-TPP NPs (11.23 N), compared with that of control group (6.03 N). The high hardness of meat products could be attributed to the reduction of bound water in the batter during the cooking process (Horita et al. 2011). For control samples, the lower hardness values might be related to the proteolytic activity of endogenous or microbial proteases and collagenases (Viji et al. 2014). In the present study, the hardness values of fish surimi were much lower than the hardness range of 5181 to 8143 g for fish burgers (Coelho et al. 2007). In fact, Nishimura (2010) reported that the hardness values are commonly associated with the amount of the stromal proteins in fish ranges from 2 to 3%. Further, it has been suggested that the differences in hardness might be further related to the presence of bonds between CS, CS-TPP NPs and myofibrillar proteins via covalent and non-covalent interactions, which improved the texture properties of surimi samples (Mao and Wu 2007). In fact, the myofibrillar protein might be affected during the cold storage, which could be translated to the quality of fish surimi, as well as the consumer acceptance.
Springiness was defined as the rate at which a deformed surimi recovers its initial conditions. The changes in springiness during the cold storage are summarized in Table 5. Results showed that CS and CS-TPP NPs addition didn't show a significant improvement of the springiness values of fish surimi. In addition, a low increase in springiness values was detected with the storage time for CS-TPP NPs treatment and attained 3.86 mm at the 9th day. It was observed an increase in adhesiveness values with CS and especially CS-TPP NPs addition onto surimi samples. Consequently, the texture of surimi samples was not deteriorated during storage time. However, textural scores for control samples were numerically lower than those of treated samples with CS and CS-TPP NPs. Thus, CS-TPP NPs could be used as a natural ingredient to maintain the fish surimi based food systems for the development of novel healthy fish products and addresses consumer demands for functional fish products.
Microbial analysis of surimi
During storage time, the microbiological quality and shelf life stability of the stored fish surimi is commonly recognized. Therefore, the mesophilic, psychrophilic bacteria, yeast and molds plate counts were investigated during the storage time and results are reported in Table 6. The initial mesophilic plate count in the different formulations supplemented with CS and CS-TPP NPs, with respective values of 3.02 and 3 log CFU/g of surimi, was similar to that of the control group (3.1 log CFU/g of surimi). The mesophilic bacteria counts increased gradually in all samples during storage time. On day 9, CS and CS-TPP NPs groups attained 5.12 and 4.98 log CFU/g of surimi, respectively, which was significantly lower than that of groups without any supplements (control group) reaching 6.45 CFU/g.
Regarding the psychrophilic bacteria plate counts, a rapidly increase from 2.10 to 6.54 log CFU/g of surimi after 9 days of storage at 4 °C was observed, as compared to groups supplemented with CS and CS-TPP NPs showing low increase in bacteria growth (Table 6). These results showed that the CS and CS-TPP NPs reduced significantly the mesophilic and psychrophilic bacteria and restricted the growth of microorganisms, which were likewise observed for the yeast and molds plate accounts.
Indeed, on 9th day, surimi groups supplemented with CS and CS-TPP NPs had total yeast and molds counts of 4.20 and 4.21 log CFU/g, which was significantly higher for the control group (5.12 log CFU/g). The lower bacterial population is correlated with the higher hardness values of enriched surimi, as above mentioned. This could be due to proteinases enzymes that are produced by spoilage microorganisms, which hydrolyze the muscle protein.
Gilbert et al. (2000) reported that a bacteria count of 5 log CFU/g was considered as acceptable limit for restructured products. Based on the microbial analysis results, the fish surimi enriched with CS and CS-TPP NPs were in acceptable level for human consumption. However, the control group reached a rejection level after 9 days of storage at 4 °C. This finding was due to the antimicrobial effects of CS and CS-TPP NPs, as previously mentioned. Results were in agreement with those reported by Georgantelis et al. (2007) who found that treatment with CS at a concentration of 0.3–0.6% and 1%, respectively, increased the shelf-life of fresh pork sausages stored at chilled temperature from 7 to 15 days.
Conclusion
CS-TPP NPs with an average size of 121–321 nm were successfully prepared using ionic gelation process. The prepared nanoparticles were characterized by DLS, FTIR, XRD and NMR spectroscopies. Thermogravimetric analysis showed that the CS-TPP NPs were thermally stable, and could be used even at elevated temperatures. CS-TPP NPs were characterized by an interesting antioxidant and antimicrobial activities against Gram-negative and Gram-positive bacteria, as well as fungal strains. As a result, the prepared nanoparticles could be employed in foods and pharmaceutical industries. Further, CS (1%) and CS-TPP NPs (1%) supplements could reduce the microbial growth and the oxidation values of surimi samples during 9 days of storage compared to the uncoated one. This suggests that CS-TPP NPs could be a viable solution to maintain the quality of fish surimi during the storage. The texture profile analysis parameters were improved by the addition of CS-TPP NPs during the cold storage. Results showed that CS-TPP NPs could be used effectively to extend the shelf life of fish surimi products under chilled storage. Thus, CS nanoparticles could be used as a natural ingredient to prevent lipid oxidation in surimi based food systems for the development of novel healthy fish products and addresses consumer demands for functional fish products.
References
Akmaz S, AdJgüzel ED, Yasar M, Erguven O (2013) The effect of Ag content of the chitosan-silver nanoparticle nomposite material on the structure and antibacterial activity. Adv Mater Sci Eng. Article ID 690918, 6
Ali WS, Joshi M, Rajendran S (2010) Modulation of size, shape and surface charge of chitosan nanoparticles with reference to antimicrobial activity. Adv Sci Lett 3:452–460
Alishahi A, Mirvaghefi A, Tehrani MR, Farahmand H, Shojaosadati SA, Dorkoosh FA, Elsabee MZ (2011) Shelf life and delivery enhancement of vitamin C using chitosan nanoparticles. Food Chem 126:935–940
Anraku M, Fujii T, Furutani N, Kadowaki D, Maruyama T, Otagiri M, Gebicki JM, Tomida H (2009) Antioxidant effects of a dietary supplement: reduction of indices of oxidative stress in normal subjects by water-soluble chitosan. Food Chem Toxicol 47:104–109
Arulmozhi V, Pandian K, Mirunalini S (2013) Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids Surf B 110:313–320
Barbi MS, Carvalho FC, Kiill CP, Barud HS, Santagneli SH, Ribeiro SJL, Gremião MPD (2015) Preparation and characterization of chitosan nanoparticles for zidovudine nasal delivery. Nanosci Nanotechnol 15:865–874
Benjakul S, Bauer F (2001) Biochemical and physicochemical changes in catfish muscle as influenced by different freeze-thaw cycles. Food Chem 72:207–217
Bertrand N, Wu J, Xu X, Kamaly N, Farokhad OC (2014) Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev 66:2–25
Calero N, Munoz J, Ramirez P, Guerrero A (2010) Flow behaviour, linear viscoelasticity and surface properties of chitosan aqueous solutions. Food Hydrocoll 24:659–666
Calvo P, Remunan Lopez C, Vila Jato JL, Alonso MJ (1997) Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 63:125–132
Chaijan M, Benjakul S, Visessanguan W, Faustman C (2004) Characteristics and gel properties of muscles from sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) caught in Thailand. Food Res Int 37:1021–1030
Chaijan M, Benjakul S, Visessanguan W, Faustman C (2005) Changes of pigments and colour in sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) muscle during iced storage. Food Chem 93:607–617
Chaijan M, Benjakul S, Visessanguan W, Faustman C (2006) Changes of lipids in sardine (Sardinella gibbosa) muscle during iced storage. Food Chem 99:83–91
Chaijan M, Panpipat W, Benjakul S (2010) Physicochemical properties and gel-forming ability of surimi from three species of mackerel caught in Southern Thailand. Food Chem 121:85–92
Chen HH (2002) Decoloration and gel-forming ability of horse mackerel mince by air-flotation washing. J Food Sci 67:2970–2975
Chi CF, Hu FY, Wang B, Ren XJ, Deng SG, Wu CW (2015) Purification and characterization of three antioxidant peptides from protein hydrolyzate of croceine croaker (Pseudosciaena crocea) muscle. Food Chem 168:662–667
Chuah LH, Billa N, Roberts CJ, Burley JC, Manickam S (2011) Curcumin-containing chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Pharm Dev Technol 18:591–599
Cioffi N, Torsi L, Ditaranto N, Tantillo G, Ghibelli L, Sabbatini L, Bleve-Zacheo T, D’Alessio M, Zambonin PG, Traversa E (2005) Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem Mater 17:5255–5262
Coelho G, Weschenfelder Â, Meinert E, Amboni R, Beirão LH (2007) Effects of starch properties on textural characteristics of fish burgers: sensory and instrumental approaches. Boletim Centro de Pesquisa de Processamento de Alimentos 25:37–50
Colmenero FJ, Barreto G, Fernández P, Carballo J (1996) Frozen storage of bologna sausages as a function of fat content and of levels of added starch and egg white. Meat Sci 42:325–332
Darmadji P, Izumimoto M (1994) Effect of chitosan in meat preservation. Meat Sci 38:243–254
de Pinho Neves AL, Milioli CC, Müller L, Riella HG, Kuhnen NC, Stulzer HK (2014) Factorial design as tool in chitosan nanoparticles development by ionic gelation technique. Colloids Surf A Physicochem Eng Asp 445:34–39
Dey SS, Dora KC, Raychaudhuri U, Ganguly S (2013) Cryoprotective effect of shrimp waste protein hydrolysate on croaker surimi protein and gel characteristics during frozen storage. Fish Technol 50:50–59
Divya K, Jisha MS (2017) Chitosan nanoparticles preparation and applications. Environ Chem Lett 16:101–112
Divya K, Vijayan S, Jisha MS (2018) Antifungal, antioxidant and cytotoxic activities of chitosan nanoparticles and its use as an edible coating on vegetables. Int J Biol Macromol 114:572–577
Du WL, Niu SS, Xu YL, Xu ZR, Fan CL (2009) Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr Polym 75:385–389
Dudhani AR, Kosaraju SL (2010) Bioadhesive chitosan nanoparticles: preparation and characterization. Carbohydr Polym 81:243–251
Esterbauer H, Cheeseman KH, Dianzani MU, Poli G, Slater TI (1982) Separation andcharacterization of the aldehydic products of lipid peroxidation stimulated by ADP Fe2+in rat liver microsomes. Biochem J 208:129–140
Fernandez-Kim SO (2004) Physicochemical and functional properties of crawfish chitosan as affected by different processing protocols. Thesis
Georgantelis D, Ambrosiadis I, Katikou P, Blekas G, Georgakis SA (2007) Effect of rosemary extract, chitosan and α-tocopherol onmicrobiological parameters and lipid oxidation of fresh pork sausages stored at 4 °C. Meat Sci 76:172–181
Gilbert RJ, de Louvois J, Donovan T, Little C, Nye K, Ribeiro CD, Richards J, Roberts D, Bolton FJ (2000) Guidelines for the microbiological quality of some ready-to-eat foods sampled at the point of sale. PHLS advisory committee for food and dairy products. Commun Dis Public Health 3:163–167
Gomez-Basauri JV, Regenstein JM (1992) Vacuum packaging, ascorbic acid and frozen storage effects on Heme and Nonheme iron content of Mackerel. J Food Sci 57:1337–1339
Gu HW, Ho PL, Tong E, Wang L, Xu B (2003) Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett 3:1261–1263
Haard NF (1992) Biochemistry and chemistry of color and color change in seafoods. Advances in Seafood Biochemistry. Technomic Publishing Co, Inc, Lancester, Penn Sylvania, pp 312–319
Hajji S, Younes I, Ghorbel-Bellaaj O, Hajji R, Rinaudo M, Nasri M, Jellouli K (2014) Structural differences between chitin and chitosan extracted from three different marine sources. Int J Biol Macromol 65:298–306
Hamdi M, Hajji S, Affes S, Taktak W, Maâlej H, Nasri M, Nasri R (2017) Development of a controlled bioconversion process for the recovery of chitosan from blue crab (Portunus segnis) exoskeleton. Food Hydrocoll 101:455–463
Hansen LJ, Sereika HE (1969) Factors affecting color stability of prepackaged frozen fresh beef in display cases. Illum Eng 64:620–624
Hebeish A, Sharaf S, Farouk A (2013) Utilization of chitosan nanoparticles as a green finish in multifunctionalization of cotton textile. Int J Biol Macromol 60:10–17
Horita CN, Morgano MA, Celeghini RMS, Pollonio MAR (2011) Physicochemical and sensory properties of reduced-fat mortadella prepared with blends of calcium, magnesium and potassium chloride as partial substitutes for sodium chloride. Meat Sci 89:426–433
Hosseini SF, Rezaei M, Zandi M, Farahmandghavi F (2015) Fabrication of bio-nanocomposite films based on fish gelatin reinforced with chitosan nanoparticles. Food Hydrocoll 44:172–182
Hosseini SF, Soleimani MR, Nikkhah M (2018) Chitosan/sodium tripolyphosphate nanoparticles as efficient vehicles for antioxidant peptidic fraction from common kilka. Int J Biol Macromol 111:730–737
Huang ZM, Zhang Y, Kotaki M, Ramakrishnaj S (2003) A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol 63:2223–2253
Jingou J, Shilei H, Weiqi L, Danjun W, Tengfei W, Yi X (2011) Preparation, characterization of hydrophilic and hydrophobic drug in combine loaded chitosan/cyclodextrin nanoparticles and in vitro release study. Colloids Surf B 83:103–107
Jung KH, Lee JH, Park JW, Quach HT, Moon SH, Cho YS, Lee KH (2015) Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. Int J Pharm 478:251–257
Kafshgari MH, Khorram M, Mansouri M, Samimi A, Osfouri S (2012) Preparation of alginate and chitosan nanoparticles using a new reverse micellar system. Iran Polym J 21:99–107
Kanatt SR, Chander R, Sharma A (2008) Chitosan and mint mixture: a new preservative for meat and meat products. Food Chem 107:845–852
Kaya M, Mujtaba M, Ehrlich H, Salaberria AM, Baran T, Amemiya CT, Galli R, Akyuz L, Sargin I, Labidi J (2017) On chemistry of γ-chitin. Carbohydr Polym 176:177–186
Ke PJ, Cervantes E, Robles-Martinez C (1984) Determination of thiobarbituric acid reactive substances (TBARS) in fish tissue by an improved distillation spectrophotometric method. J Sci Food Agric 35:1248–1254
Kim DG, Jeong YI, Choi C, Roh SH, Kang SK, Jang MK, Nah JW (2006) Retinol-encapsulated low molecular water-soluble chitosan nanoparticles. Int J Pharm 319:130–138
Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, Cho CS (2008) Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 26:1–21
Kim JH, Kim SI, Kwon IB, Kim MH, Lim JI (2013) Simple fabrication of silver hybridized porous chitosan-based patch for transdermal drug-delivery system. Mater Lett 95:48–51
Koleva II, Van Beek TA, Linssen JPH, De Groot A, Evstatieva LN (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal 13:8–17
Lee S, Joo ST, Alderton AL, Hill DW, Faustman C (2003) Oxymyoglobin and lipid oxidation in yellow fin tuna (Thunnus albacares) loins. J Food Sci 68:1664–1668
León FJC, Lizardi-Mendoza J, Arguelles-Monal W, Carvajal-Millan E, Lopez Franco YL, Goycoolea FM (2017) Supercritical CO2 dried chitosan nanoparticles: production and characterization. RSC Adv 7:30879–30885
Li Y, Song H, Xiong S, Tian T, Liu T, Sun Y (2005a) Chitosan-stablized bovine serum albumin nanoparticles having ability to control the release of NELL-1 protein. Int J Biol Macromol 109:672–680
Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M (2005b) Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials 26:3919–3928
Maciel VBV, Yoshida CMP, Pereira SMSS, Goycoolea FM, Franco TT (2017) Electrostatic self-assembled chitosan-pectin nano- and microparticles for insulin delivery. Molecules 22:1707
Mao L, Wu T (2007) Gelling properties and lipid oxidation of kamaboko gels from grass carp (Ctenopharyngodon idellus) influenced by chitosan. J Food Eng 82:128–134
Meshkini S, Tafy AA, Tukmechi A, Farhang-Pajuh F (2012) Effects of chitosan on hematological parameters and stress resistance in rainbow trout (Oncorhynchus mykiss). Vet Res Forum 3:49–54
Nallamuthu I, Devi A, Khanum F (2015) Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J Pharm Sci 10:203–211
Nguyen TV, Nguyen TTH, Wang SL, Khanh Vo TP, Nguyen AD (2017) Preparation of chitosan nanoparticles by TPP ionic gelation combined with spray drying, and the antibacterial activity of chitosan nanoparticles and a chitosan nanoparticle–amoxicillin complex. Res Chem Intermed 43:3527–3537
Nishimura T (2010) The role of intramuscular connective tissue in meat texture. Animal Sci J 81:21–27
Ochiai Y, Ochiai L, Hashimoto K, Watabe S (2001) Quantitative estimation of dark muscle content in the mackerel meat paste and its products using antisera against myosin light chains. J Food Sci 66:1301–1305
Ocloo FCK, Quayson ET, Adu-Gyamfi A, Quarcoo EA, Asare D, Serfor Armah Y, Woode BK (2011) Physicochemical and functional characteristics of radiation-processed shrimp chitosan. J Radiat Phys Chem 80:837–841
Phromsopha T, Baimark Y (2010) Chitosan microparticles prepared by the water-in-oil emulsion solvent diffusion method for drug delivery. Biotechnology 9:61–66
Piyakulawat P, Praphairaksit N, Chantarasiri N, Muangsin N (2007) Preparation and evaluation of chitosan/carrageenan beads for controlled release of sodium diclofenac. AAPS Pharm Sci Technol 8:120–130
Plainsirichai M, Leelaphatthanapanich S, Wongsachai N (2014) Effect of chitosan on the quality of rose apples (Syzygium Agueum Alston) cv. Tabtim Chan stored at an ambient temperature. APCBEE Proc 8:317–322
Postnikova GB, Tselikova SV, Kolaeva SG, Solomonov NG (1999) Myoglobin content in skeletal muscles of hibernating ground squirrels rises in autumn and winter. Comp Biochem Physiol Part B 124:35–37
Qi L, Xu Z, Jiang X, Hu C, Zou X (2004) Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res 339:2693–2700
Rajalekshmi M, Mathew PT (2007) Colour and textural parameters of threadfin bream surimi during frozen storage as affected by cryoprotectants and chitosan. Fish Technol 44:55–64
Rodrigues S, Costa AM, Grenha A (2012) Chitosan/carrageenan nanoparticles: effect of cross-linking with tripolyphosphate and charge ratios. Carbohydr Polym 89:282–289
Rokhade AP, Agnihotri SA, Patil SA, Mallikarjuna NN, Kulkarni PV, Aminabhavi TM (2006) Semi-interpen etrating polymer network microspheres of gelatin and sodium carboxymethyl cellulose for controlled release of ketorolac tromethamine. Carbohydr Polym 65:243–252
Ruan S, Yuan M, Zhang L, Hu G, Chen J, Cun X, Gao H (2015) Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 37:425–435
Shahidi F, Arachchi JKV, Jeon YJ (1999) Food applications of chitin and chitosans. Trends Food Sci Technol 10:37–51
Tokumitsu H, Ichikawa H, Fukumori Y (1999) Chitosan-gadopentetic acid complex. Nanoparticles for gadolinium neutron-capture therapy of cancer: preparation by novel emulsion-droplet coalescence technique and characterization. Pharm Res 16:1830–1835
Vasconcelos F, Cristol S, Paul JF, Tricot GG, Amoureux JP, Montagne L, Mauri F, Delevoye L (2008) 17O solid-state NMR and first-principles calculations of sodium trimetaphosphate (Na3P3O9), tripolyphosphate (Na5P3O10) and pyrophosphate (Na4P2O7). Inorg Chem 47:7327–7337
Viji P, Tanuja S, Ninan G, Zynudheen AA, Lalitha KV (2014) Quality characteristics and shelf life of sutchi cat fish (Pangasianodon hypophthalmus) steaks during refrigerated storage. Int J Agric Food Sci Technol 5:105–116
Wang YF, Huang SR, Shao SH, Qian L, Xu P (2012) Studies on bioactivities of tea (Camellia sinensis L.) fruit peel extracts: antioxidant activity and inhibitory potential against α-glucosidase and α-amylase in vitro. Ind Crops Prod 37:520–526
Yang HC, Wang WH, Huang KS, Hon MH (2010) Preparation and application of nanochitosan to finishing treatment with anti-microbial and anti-shrinking properties. Carbohydr Polym 79:176–179
Yen GC, Wu J (1999) Antioxidant and radical scavenging properties of extracts from Ganoderma tsugae. Food Chem 65:375–379
Yildirim A, Mavi A, Kara AA (2001) Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem 49:4083–4089
Acknowledgments
This work was supported by Grants from Ministry of Higher Education and Scientific Research of Tunisia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hajji, S., Hamdi, M., Boufi, S. et al. Suitability of chitosan nanoparticles as cryoprotectant on shelf life of restructured fish surimi during chilled storage. Cellulose 26, 6825–6847 (2019). https://doi.org/10.1007/s10570-019-02555-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02555-1