Abstract
A novel durable intumescent flame retardant coating, based on metal oxide nanoparticles (NPs) and biomacromolecules, was designed and applied on cotton fabrics. Specifically, different TiO2 NPs/proteins systems were deposited by dip-pad-dry-cure process and the morphology of the resulting coating were assessed by SEM analysis. Enhancement of durability (i.e. resistance to washing treatments) was verified by release tests carried out in static and dynamic conditions. Flammability and cone calorimetry tests were performed for evaluating the fire behavior of the treated fabrics. More specifically, in horizontal flame spread tests, the different nanoparticle/protein based coatings provided an increase of the total burning time and a decrease of the burning rate. Furthermore, the residues at the end of the test were significantly higher with respect to untreated cotton fabric. In particular, casein-based systems seemed to be more effective as compared to the whey proteins counterparts. Cone calorimetry tests showed better fire performances for the coatings based on TiO2/caseins with respect to TiO2/whey proteins, which did not seem to be so effective in protecting the underlying fabric from the heat flux. Therefore, due to their high char-forming character, casein-based coatings may represent an effective and durable fire-resistant finishing alternative to standard flame retardant treatments for cotton.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Burning of textiles has always been considered a major hazard: in fact, most fibers and fabrics, which play an important role in everyday life (e.g. for transportation, automotive industry, protective garments, military, furniture upholstery, bed linen and nightwear), are flammable and potentially dangerous materials (Alongi et al. 2013). As a consequence, chemical species (so-called flame retardants—FRs) have been developed to limit the risk of fire by inhibiting fabric ignition or, at least, to reduce the rate of flame spread. Thus, flame retardant finishing can be considered among the most important textile finishing methods, and is undoubtedly the noblest technical treatment. In the recent years, the issue of textile flammability has encouraged academic and industrial researchers to explore novel synthetic strategies inherent to flame retardant finishing systems. The fields of interest are numerous and finalized to guarantee human safety. Indeed, great attention has been focused worldwide on reducing the hazard of fabric burning through different directives and standards. Nowadays, the challenge for researchers is the search of environmentally-friendly FRs that exhibit remarkable “green” character and ensure fire performances comparable to those achieved by conventional FR compounds, which have been usually designed on the basis of halogens or halogen-derivatives (Horrocks and Price 2008). Among the currently commercially-available FRs, phosphorus and nitrogen-based compounds may represent a suitable alternative to their environmentally unfriendly halogen-based counterparts. Some considerably successful examples of intumescent FR materials have been reported quite recently (Alongi et al. 2014). The term “intumescence” is generally used to describe the behavior of some materials having FR features, in particular plastics or textiles. Upon heating, intumescent materials begin to swell and then to expand: as a consequence, a foamy carbonaceous residue (char) is formed on the surface, which protects the underlying material from the heat flux or the flame. Intumescent coating chemistry has been thoroughly described by Vandersall in a widely known review (Vandersall 1971). An intumescent material consists of three main components: (1) an acid-generating species, most commonly ammonium polyphosphate (APP); (2) a carbon source; (3) a blowing agent (i.e. melamine, guanidine, etc.), which generates gaseous products (usually N2 or carbon dioxide) in the molten mass, giving rise to the formation of a solid foam that acts as a heat- and vapor-transfer barrier.
Biomacromolecules (among which whey proteins and caseins) are unusual green alternatives to the conventional intumescent FRs (Malucelli et al. 2014). The use of whey proteins and caseins as FRs offers significant advantages, being environmentally-friendly and being caseins and whey proteins easily recoverable from waste or by-products of the dairy industry. The main disadvantage for biomacromolecule coating is their low washing fastness, resulting in FR effect loss (Malucelli et al. 2014). Nowadays, the challenge for researchers is the search for effective FR finishing systems with appropriate washing fastness. Recently, some progress has been made by Casale et al. (2016), who were able to increase the washing fastness of a FR biomacromolecule coating (consisting of deoxyribonucleic acid, DNA, and chitosan) deposited on cotton fabrics, exploiting a photo-induced cross-linking strategy.
The majority of literature reports on the production of functionalized nanomaterials for biotechnological and biomedical applications, such as nanocarriers in drug delivery, contrast agents in molecular imaging, and diagnostic agents in cancer therapy (Sapsford et al. 2013; Bishop et al. 2016; Cai et al. 2017). The use of biomacromolecules as FR additives has mostly been limited to the design of fire-protective coatings on different fabric substrates (namely, cotton, polyester and their blends) using either an impregnation/exhaustion (Malucelli et al. 2014) or a layer-by-layer (LbL) approach (Alongi et al. 2012). In the latter case, it was found it possible to exploit the presence of the inorganic phase within the LbL assembly for enhancing the flame retardant effect provided by the biomacromolecule through the formation of a ceramic protective layer upon exposure of the treated substrate to a flame or heat source. Conversely, to the best of the authors’ knowledge, the scientific literature lacks significant instances describing the flame retardant behavior of coatings obtained by combining two completely different phases (i.e. an inorganic phase and biomacromolecules) into an all-in-one stable aqueous dispersion, to be applied to the fabric substrate through the impregnation/exhaustion method.
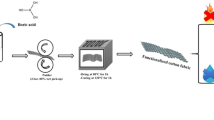
Therefore, in this work, we thoroughly investigate the FR effects derived from the coupling an inorganic phase (namely, nano-TiO2) with selected biomacromolecules (whey proteins and caseins), trying to assess the occurrence of possible joint or synergistic effects between the two components, which may enhance the fire-retardant properties of the treated cotton fabrics.
In particular, we investigated the introduction of a nano-TiO2 (inorganic) phase already applied to textile substrates for the production of photocatalytic ceramic coating (Ortelli et al. 2014, 2015) for its capability to thermally insulate the substrate, enhance char formation and act as a cross-linking platform for targeting intumescent molecules on textile surface, hence improving their stability and adhesion.
Experimental
Materials
TiO2 colloidal nanosuspension containing 6 wt% TiO2, was provided by Colorobbia (Italy). Sodium citrate (CIT) and Dowex® 66 anion exchange resin were purchased from Sigma-Aldrich (Italy). Nitric acid (65%) was purchased from Titolchimica S.p.A. (Italy). Whey proteins powder (93.5 wt% protein) was purchased from Anderson Research (Cervaro (FR), Italy); its composition also includes lipids (ca. 0.5 wt%), carbohydrates (ca. 1 wt%), ash (ca. 2.2 wt%), and moisture (ca. 2.8 wt%). Amino acid content is 89.5 wt%, as indicated in the supplier’s data sheet.
Caseins (reagent grade) were purchased from Sigma-Aldrich (Italy); their approximate composition is as follows: 12–15 α-s1, 3–4 α-s2, 9–11 β and 2–4 κ (concentrations in g/L, as stated in the product information sheet).
Preparation of suspensions
3% w/v whey proteins (WP) solution was prepared by dissolving powder in distilled water under magnetic stirring for 1 h at room temperature. The so-obtained whey proteins solution had pH = 5.5. Caseins solution (3% w/v) was prepared by dissolving powder in aqueous NaOH to final pH = 8, under magnetic stirring for 15 h at T = 80 °C. We used two different methods to raise the very low pH of commercial TiO2 nanosol. Firstly, we used a weak anionic exchange resin to obtain the sample called TACR with final pH = 4, as described in our previous work (Ortelli et al. 2015). In the second method we slowly added sodium citrate (CIT) to TiO2 nanosol (TiO2:CIT weight ratio 1:0.83) to reach a final pH = 6 value after 15 h of mechanical stirring. Whey protein or casein solutions were added to TiO2 nanosuspension to a TiO2:proteins weight ratio equal to 0.7:1. The ratio was optimized by titrating the TiO2 NPs with proteins solution while measuring the Z potential, using a Zetasizer Nanoseries—Malvern Instruments, Malvern, UK apparatus coupled with automatic titrating system, calculating the amount corresponding to the beginning of the plateau of the Z potential curve vs. amount of protein added. More details are reported in Supporting Information (Figure S1).
Cotton fabric samples
Cotton fabrics (200 g/m2) were purchased from Fratelli Ballesio S.r.l. (Torino, Italy). Before treatment, the fabrics were ultrasonicated in water for 30 min at room temperature. The TiO2/proteins suspensions were applied to the cotton surface by the traditional dip-pad-dry-cure method. More specifically, the fabrics were dipped into two different nano-TiO2/proteins suspensions, namely TACR/WP and TiO2CIT/caseins and left to soak for 3 min, then passed through a two-roller laboratory padder, oven dried at 100 °C, and finally cured for 10 min at 130 °C. Multiple impregnations were carried out to achieve the final dry add-on value (AO%), defined as the percent amount of the finishing agent added to the fabric with respect to the initial weight of the latter, i.e.
where wi and wf are the weights of the fabric before and after the dip-pad-dry-cure process. A Sartorius balance (± 10−4 g resolution) was used to measure wi and wf.
Two sets of samples at about 8 and 16 AO% were prepared by performing 4 and 8 impregnation steps, respectively. The experimental AO% values obtained are reported in Table S1. Monophasic (pure TACR, TiO2CIT, WP and caseins) coated cotton fabrics were produced and used as control samples.
Samples characterization
Characterization of suspensions
In order to study the colloidal behavior and the affinity between inorganic and biomacromolecules phases, Zeta potential versus pH measurements were carried out by titration on the TACR/WP and TiO2CIT/casein systems using a Zetasizer Nanoseries (Malvern Instruments, Malvern, UK) instrument coupled with an automatic titrating system. The titration was performed with 1 M KOH and 1 M HCl solutions on proteins/TiO2 NPs mixtures diluted to 1:10 v/v with distilled water. Zeta potential-pH curves were used to identify the isoelectric point, pHi.e.p., i.e. the pH at which zeta potential sets to zero (pHi.e.p.).
Morphological characterization of cotton fabric samples
The presence and the morphology of nano-TiO2/protein-based coatings on cotton fabrics were assessed by scanning electronic microscopy analysis using a Carl Zeiss Sigma NTS (Gmbh Öberkochen, Germany) Field Emission Scanning Electron Microscope (FE-SEM). Uncoated and coated cotton fabrics (about 3 mm × 3 mm) were cut and fixed to conductive adhesive tapes and sputter-metallized with gold.
Washing fastness: release test
The release of TiO2 NPs and proteins from treated cotton samples was assessed by dipping fabrics in distilled water, both in static and dynamic conditions. The release tests were performed on fabrics treated either with each single component (i.e. TiO2 NPs, whey proteins and caseins alone) or with the nanoparticle/biomacromolecule suspensions. The static test was carried out by immersing treated cotton samples (25 × 25 mm2) in 6 mL MilliQ water for up to 8 days, while samples of washing water were collected at defined intervals (i.e. after 1, 4 and 6 days). For each analysis time, a different fabric sample was used. On the other hand, the dynamic test was carried out by immersing the treated cotton samples (50 × 50 mm2) in 25 mL MilliQ water, in an ultrasound (US) bath for 10 min for 3 cycles; the same sample was subjected to 3 US cycles. To determine TiO2 and proteins contents, the collected samples of washing water obtained in static and dynamic conditions were analyzed by inductively coupled plasma optical emission spectrometry using an ICP-OES 5100—vertical dual view apparatus (Agilent Technologies, Santa Clara, CA, USA). The analysis was performed in axial viewing mode, and calibration curves were obtained with 0.1, 1.0, 10.0, 100.0 and 300.0 ppm standards for every analyzed element. Nitric acid was added both to standards and to diluted samples (1:10 v/v). The concentration of TiO2 in washing water was directly evaluated by ICP-OES Ti determination, whereas calibration curves were obtained to estimate proteins concentration. In order to extrapolate protein concentration in washing waters, calibration curves were obtained by plotting whey proteins and caseins concentration as a function of S and P content, respectively. All calibration curves were evaluated and showed correlation coefficients (R2) above 0.99. Results from ICP-OES were reported as the average of three independent measurements with relative standard deviation (rsd%).
Flammability tests
Flame spread tests in horizontal configuration (according to ASTM D4986) were carried out by applying a 25 mm methane flame for 3 s to the short side of 50 × 100 mm2 specimens. These tests were repeated 3 times for each formulation. Total burning time and burning rate following flame application were measured, as well as the amount of final residue.
Cone calorimetry tests
The combustion behavior of square fabric samples (50 × 50 mm2) was investigated by cone calorimetry (Fire Testing Technology, FTT). Measurements were carried out under a 35 kW/m2 radiative heat flow in horizontal configuration, following the procedure described elsewhere (Tata et al. 2011). Such parameters as Time To Ignition (TTI, s), peak of Heat Release Rate (PHRR, kW/m2g) and Total Heat Release (THR, kJ/m2) were measured. The experiments were repeated four times for each material investigated to check reliability and reproducibility of data, which yielded experimental errors within 5%.
Prior to flammability and combustion tests, all the specimens were conditioned at 23 ± 1 °C for 48 h at 50% R.H. in a climatic chamber.
Results and discussion
In order to make a durable immobilized FR coating on fabric, we exploited the ability of aminoacidic biomacromolecules to irreversibly coat nanoparticles (NPs) according to the protein corona theory (Lynch and Dawson 2008; O’Connell et al. 2015), as well as the affinity of metal oxide NPs towards naturally hydrophilic fibers, as demonstrated by ceramization processes reported in our previous works (Ortelli et al. 2014, 2015). The design strategy adopted in this work is schematically shown in Fig. 1.
Characterization of suspensions
In order to study the colloidal behavior and the affinity between inorganic and biomacromolecules phases, Zeta potential versus pH titrations were performed (Fig. 2). The TiO2 colloidal suspension (TACR) displays positive Zeta potential in the acidic pH range, with a pHi.e.p. of 7.0. On the other hand, the WP solution has a negative Zeta potential at natural pH (pH = 5.5), with a pHi.e.p. of 3.8. This findings justify the covering of TiO2 NPs by whey proteins, due to the strong electrostatic interactions between positively charged TiO2 NPs and negatively charged proteins. This is further confirmed by the significant shift of TACR/WP mixtures’ pHi.e.p. towards the acidic pH of whey proteins pHi.e.p.. To avoid strong colloidal destabilization due to a large pH difference (Pfeiffer et al. 2014), caseins solution (pH = 8) was added to citrate-modified TiO2 NPs (pH 6), which show negative Zeta potential in the whole pH range (Fig. 2b, red curve). In this case, at the starting pH of the two phases, the electrostatic interactions are weaker, due to the absence of oppositely charged surfaces. Despite this, the high correspondence found in TiO2 and caseins pHi.e.p, again points out the likely covering of TiO2 NPs by protein. The main data from zeta potential measurements are summarized in Table 1.
Morphological characterization of cotton fabric samples
The presence of coating on cotton fabrics was confirmed by both add-on values, which were found to be proportional to the number of applied layers (data reported in Table S1), and FE-SEM images (Fig. 3). FE-SEM analysis shows the surface morphology change induced by two different nano-TiO2/protein based coatings. Unlike the smooth texture of the uncoated fiber (Fig. 3a, b), the coated fibers (Fig. 3c–f) show a certain surface roughness due to the presence of nano-TiO2/protein layers attached on cotton substrate fibers. Slight inhomogeneity and presence of large aggregates, particularly visible in TACR/WP coated fibers (Fig. 3c, d) is probably caused by the multiple layers applied on the cotton substrates, which favor an “island growth” of the coating.
Washing fastness: release tests
In order to specifically evaluate the interactions between proteins and biomacromolecules and their affinity with the cotton substrate, we assessed the release of TiO2 and protein, whey protein and casein concentrations being estimated by S and P content, respectively, using the calibration curves shown in Figure S2. In addition, the release behavior of the single phases, TiO2 NPs and biomacromolecules alone (both whey proteins and caseins), was investigated using control samples. All the obtained data are reported in Figs. 4, 5 and Tables S2–S5.
As regards monophasic samples (i.e. TiO2 NPs and biomacromolecules alone), we observed that TACR, as expected (Costa et al. 2013), exhibits good adhesion onto the cotton substrate, showing very low percent weight loss values. A strong decrease of adhesion is found for the TiO2CIT coating, which can be ascribed to the presence of citrate around TiO2 NPs that hinders their electrostatic interactions with the cotton fibers (Figs. 4a, 5a). As expected (Malucelli et al. 2014), the pure biomacromolecule coatings generally show high percent weight loss: in particular, those based on pure caseins present values corresponding to a complete release (weight loss 100%) in static condition, (see Fig. 5c and Table S5).
As regards biphasic coatings (i.e. WP and caseins in the presence of TiO2 NPs), all samples showed a lower biomacromolecules release with respect to the pure WP- and casein-based coatings, thus confirming the presence of a strong interaction between the two organic/inorganic coupled phases and the cotton substrate. During the dynamic tests, after the first cycle, the released amount dropped very quickly; furthermore, in static condition, the initial released amount reached a plateau in few hours, and did not change afterwards. Finally, it is noteworthy that both dynamic and static conditions agree with a kinetics of biomacromolecules release at short time, as already reported in the literature (Casale et al. 2016). In addition, a very interesting and surprising result comes from the comparison of TiO2 NPs washing fastness in the presence or in the absence of casein. More specifically, the TiO2CIT/casein coatings show an improvement in adhesion onto cotton fiber, as proved by a dramatic decrease of % weight loss after 1 cycle, clearly shown in Fig. 5a, c, respectively, for TiO2 and proteins, hence confirming the strong interaction also occurring between both negatively charged TiO2CIT NPs and caseins.
Fire behavior
In order to describe a realistic fire scenario, it is necessary to test both the ignitability of a sample in the presence of a flame (i.e. its flammability), and the combustion behavior of the same sample under radiative heat flow. Thus, the flame retardant features of cotton fabrics, either untreated or treated with the different nanoparticle/biomacromolecule suspensions, have been assessed by means of horizontal flame spread tests, as well as forced combustion tests by cone calorimetry. Table 2 and Fig. 6 summarize the flammability results.
The application of a methane flame for 3 s to the untreated fabrics produced vigorous and quick combustion of the specimen. At the end of the tests, no residue could be found (Table 2). On the other hand, as a general trend, treatment of the fabrics with the different nanoparticle/biomacromolecule suspensions allowed increasing the total burning time while decreasing the burning rate. This clearly indicates that, even though the treated fabrics cannot achieve self-extinction, the deposited coatings are able to provide a certain protection of the underlying cellulosic substrate from flame spread, slowing down flame propagation. Furthermore, the residues at the end of the test are significantly higher as compared to the untreated fabric, especially for the systems containing caseins. It is noteworthy that the protection provided by the coatings is strictly related to their composition as well as the number of deposited layers. In particular, the coating composition being the same, the resistance towards the flame propagation increases as the number of deposited layers increases. In addition, caseins-based systems seem to be more effective than the whey proteins-based counterparts: this finding could be ascribed to the higher char-forming character of caseins with respect to WP (Malucelli et al. 2014).
Furthering this research, the resistance of the fabrics towards heat flux has been assessed by means of forced combustion tests using the cone calorimeter. The obtained results are summarized in Table 3.
First of all, it is worthy to note that fabrics coated with TiO2CIT/caseins show a better behavior with respect to untreated cotton and cotton treated with whey proteins. In fact, HRR, pkHRR and THR decrease when the fabric is coated by 4 layers of TiO2CIT/caseins; furthermore, 8 layers of the same coating are able to block the ignition of the sample, leaving, at the same time, a substantial amount of residue at the end of the test, as compared with untreated cotton. Conversely, whey proteins-based coatings do not seem to be effective in protecting the underlying fabric from the heat flux, as revealed by the increase of HRR, pkHRR and THR, despite a significant increase of the final residue. As observed in flammability tests, these findings can be ascribed to higher char formation effectiveness of caseins with respect to WP.
Conclusions
The present work introduces simple innovative design solution for the production of environmentally-friendly intumescent hybrid coatings, making a significant step forward towards the obtainment of flame-resistant textiles with improved washing fastness. More specifically, in the hybrid organic–inorganic coatings, apart from the protective effect during the exposure to a flame or a heat flux, TiO2 nanoparticles act as specific cross-linking agents, able to fix whey proteins and caseins to cotton fabrics in a stable way. The high affinities developed between biomacromolecules and NPs, as well as between NPs and the hydroxyl-rich cotton surface, enhance the washing fastness of protein-based coatings, allowing the production of durable flame retardant coatings, in addition to the benefits of using proteins as possible sustainable and eco-friendly flame retardant additives.
The encouraging anti-fire properties obtained indicate that the proposed idea could represent a novelty in the field of flame retardant treatments for cellulosic textiles.
References
Alongi J, Carosio F, Malucelli G (2012) Layer by layer complex architectures based on ammonium polyphosphate, chitosan and silica on polyester-cotton blends: flammability and combustion behaviour. Cellulose 19:1041–1050. https://doi.org/10.1007/s10570-012-9682-8
Alongi J, Horrocks RA, Carosio F, Malucelli G (2013) Update on flame retardant textiles: state of the art, environmental issues and innovative solutions. Smithers Rapra, Shropshire
Alongi J, Cuttica F, Blasio AD et al (2014) Intumescent features of nucleic acids and proteins. Thermochim Acta 591:31–39. https://doi.org/10.1016/j.tca.2014.06.020
Bishop CJ, Liu AL, Lee DS et al (2016) Layer-by-layer inorganic/polymeric nanoparticles for kinetically controlled multigene delivery. J Biomed Mater Res A 104:707–713. https://doi.org/10.1002/jbm.a.35610
Cai Z, Zhang H, Wei Y, Cong F (2017) Hyaluronan-inorganic nanohybrid materials for biomedical applications. Biomacromol 18:1677–1696. https://doi.org/10.1021/acs.biomac.7b00424
Casale A, Bosco F, Malucelli G et al (2016) DNA-chitosan cross-linking and photografting to cotton fabrics to improve washing fastness of the fire-resistant finishing. Cellulose 23:3985. https://doi.org/10.1007/s10570-016-1106-8
Costa AL, Ortelli S, Blosi M et al (2013) TiO2 based photocatalytic coatings: from nanostructure to functional properties. Chem Eng J 225:880–886. https://doi.org/10.1016/j.cej.2013.04.037
Horrocks AR, Price D (2008) Advances in fire retardant materials. Woodhead Publishing, Cambridge
Lynch I, Dawson KA (2008) Protein-nanoparticle interactions. Nano Today 3:40–47. https://doi.org/10.1016/S1748-0132(08)70014-8
Malucelli G, Bosco F, Alongi J et al (2014) Biomacromolecules as novel green flame retardant systems for textiles: an overview. RSC Adv 4:46024–46039. https://doi.org/10.1039/C4RA06771A
O’Connell DJ, Bombelli FB, Pitek AS et al (2015) Characterization of the bionano interface and mapping extrinsic interactions of the corona of nanomaterials. Nanoscale 7:15268–15276. https://doi.org/10.1039/C5NR01970B
Ortelli S, Blosi M, Albonetti S et al (2014) TiO2 based nano-photocatalysis immobilized on cellulose substrates. J Photochem Photobiol Chem 276:58–64. https://doi.org/10.1016/j.jphotochem.2013.11.013
Ortelli S, Costa A, Dondi M (2015) TiO2 nanosols applied directly on textiles using different purification treatments. Materials 8:7988–7996. https://doi.org/10.3390/ma8115437
Pfeiffer C, Rehbock C, Huhn D et al (2014) Interaction of colloidal nanoparticles with their local environment: the (ionic) nanoenvironment around nanoparticles is different from bulk and determines the physico-chemical properties of the nanoparticles. J R Soc Interface 11:20130931. https://doi.org/10.1098/rsif.2013.0931
Sapsford KE, Algar WR, Berti L et al (2013) Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem Rev 113:1904–2074. https://doi.org/10.1021/cr300143v
Tata J, Alongi J, Carosio F, Frache A (2011) Optimization of the procedure to burn textile fabrics by cone calorimeter: part I. Combustion behavior of polyester. Fire Mater 35:397–409. https://doi.org/10.1002/fam.1061
Vandersall HL (1971) Intumescent coating systems, their development and chemistry. J Fire Flammabl 2:97–140
Acknowledgments
The authors thank the European COST Action FLARETEX (MP1105) “Sustainable flame retardancy for textile and related materials based on nanoparticles substituting conventional chemicals”.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ortelli, S., Malucelli, G., Cuttica, F. et al. Coatings made of proteins adsorbed on TiO2 nanoparticles: a new flame retardant approach for cotton fabrics. Cellulose 25, 2755–2765 (2018). https://doi.org/10.1007/s10570-018-1745-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-1745-z