Abstract
This article focuses on the development of a durable multi-functional finish for cotton fabric by direct synthesis of nano zinc oxide (nano-ZnO) particles in the pores of the fibres. Anions and cations of zinc nitrate interacted with cellulose molecules and opened up the internal pore structure of cellulosic fibrils that in turn acted as nucleating sites for the formation of nano-ZnO on reaction with sodium hydroxide. The finished fabric was characterized using XRD, FTIR, SEM, TEM, AAS, DSC and AFM. The finished fabric displayed excellent antibacterial activities (>99%) against two pathogens, Staphylococcus aureus (Gram-positive) and Klebsiella pneumoniae (Gram-negative) with 40 UPF even after 30 washes confirming the utility of this method. Inherent physical and mechanical properties of the fabric were found to be unaffected. Low stress mechanical properties evaluated by Kawabata Evaluation System for Fabrics indicated that the total hand value of the finished fabric changed from 3.54 to 3.40. A plausible mechanism for in situ formation of nano-ZnO and its durability on cotton fabric has been proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton textiles are widely used for apparel due to their good feel and comfort. However, with the growing awareness of health and hygiene, they are also required to satisfy certain functional properties like antibacterial, UV (Ultraviolet) protection, etc. The advent of nano science and nanotechnology has opened a new frontier in the realm of textile finishing, i.e. nanofinishing for imparting various functional properties to the cotton materials (Vigneshwaran et al. 2006). Nano sized titanium dioxide (TiO2), zinc oxide (ZnO), silver (Ag) and copper (Cu) have been researched to impart various high functional properties like antibacterial, UV absorption and photo catalytic properties (Kathirvelu et al. 2009). Nano-ZnO has gained unique importance and scored over others due to its inertness, whiteness and cost effectiveness. ZnO has a long history of safe use and is known as an anti-irritant, skin-healing agent (Baldwin et al. 2001). It has the broadest spectrum of ultraviolet (UV) absorption (Arputharaj et al. 2016). It is well established that the application of nano-ZnO on cotton textiles improves UV protective and antibacterial properties (Saif et al. 2015; Subash et al. 2012). However, the main problem that accompanies the application of metal nanoparticles to textile materials via coating is the agglomeration of nanoparticles (Shaheen et al. 2016). Application of nano-ZnO on cotton fabric is mostly carried out with the help of polymeric binders using pad-dry-cure method (Vigneshwaran et al. 2006). This method is associated with various limitations like inducing high stiffness and low air permeability in the fabric (Gokarneshan et al. 2015). Moreover, this method of application may result in poor wash durability due to surface application. Zinc oxide cannot have any ionic interaction with the –OH groups of cellulose. So there are no possibilities for the formation of chemical bonding between cellulose and ZnO. ZnO is physically entrapped in cotton fibrillar structure. Textile materials used in technical textile applications such as hospital bed sheets undergo frequent vigorous washing. Hence, surface application of nanomaterials is not a suitable option to fulfil the high demand of wash durability. Alternative method is the in situ generation of nanoparticles with the help of precursors on the textile material. Research attempts have been reported for the in situ synthesis of nano-ZnO on cotton fabric (Perelshtein et al. 2009; Khosravian et al. 2015). However, the reported methods are tedious and consume more time, which affect the commercial exploitation of these techniques. Also, there is no detailed study available about the leaching out of zinc during laundering process of in situ finished nano ZnO cotton fabric. In this work, a simple protocol was developed to impart durable functional finishing to woven cotton fabric by in situ synthesis of nano-ZnO using Zinc nitrate hexahydrate (Zn(NO3)2·6H2O) and sodium hydroxide (NaOH) in methanol medium and the performance of the treated cotton fabrics was assessed using various instrumental techniques.
Experimental details
Materials
Plain woven grey cotton fabric (32 Ne warp count, 32 Ne weft count, 79 ends per inch, 70 picks per inch and 122 g/m2) was used in this study. The fabric was enzymatically desized using 0.5 g/l amylase, 0.5 g/l wetting agent at 70 °C for 1 h. The fabric was then scoured and bleached by following one bath method in an autoclave using 3 g/l sodium hydroxide, 6 g/l hydrogen peroxide (50% w/w), 2 g/l peroxide stabiliser and 0.2 g/l wetting agent at 120 °C for 1 h with 1:15 material to liquor ratio (MLR). Zn(NO3)2·6H2O was purchased from Sigma Aldrich®. Analytical grade methanol, NaOH and acetic acid were used for the experiment.
In situ synthesis of nano-ZnO onto cotton fabric
Cotton fabric was treated with methanolic solution of different concentrations (Table 1) of Zn(NO3)2·6H2O using 1:15 MLR at room temperature for 8 min. The fabric was padded for 80% expression and air dried. Further, it was treated with methanolic solution of NaOH using 1:15 MLR at room temperature for 8 min. The fabric was then air dried, washed with water followed by washing with dilute acetic acid solution (0.5 g/l) at room temperature. Later, the fabric was dried at 100 °C for 3 min and cured at 160 °C for 3 min in laboratory stenter (M/s RB Electronics, Mumbai). For comparative purpose, methanol was replaced by 100% aqueous and 1:1 water: methanol system and the treatments were carried out using optimised concentrations of the precursors.
Determination of antibacterial activity
To evaluate the antibacterial property of nano-ZnO treated cotton fabrics quantitatively, AATCC test method 100-2004 was used. It uses two pathogens, Staphylococcus aureus (ATCC 6538), a Gram-positive bacterium and Klebsiella pneumoniae (ATCC 4352), a Gram-negative bacterium for evaluation. One ml each of the bacterial inoculums in a growth media were fully absorbed into the fabric samples without leaving any free liquid. After incubating, the inoculated fabrics were placed in sealed jars at 37 °C for 24 h, the bacteria in the fabric were eluted and the total number of colony forming units was determined by serial dilution and plating on nutrient agar plates. Antibacterial activity, expressed as percentage reduction, was calculated by comparing the size of the initial and final bacterial population.
Determination of UV protection factor (UPF)
The ability of a fabric to block UV radiation is represented by UPF values that were determined with the help of Labsphere® instrument as per AATCC method 183-2010. Samples were conditioned at 21 ± 1 °C, 65 ± 2% RH, for 4 h before testing.
FTIR, SEM, AFM and TEM analyses
The Fourier transformation infrared (FTIR) spectra of nano-ZnO treated cotton fabrics were recorded in a Shimadzu IR-Prestige-21® using Potassium Bromide (KBr) pelleting method in the spectral range of 4000–400 cm−1. Cotton fabric was cut and powdered in a grinding mill, mixed with KBr in the ratio of 1:100 for dilution and the spectra were recorded up to 64 scans with a resolution of 2 cm−1. The contribution of the background was also accounted for. The surface of both control and nano-ZnO treated samples was observed using a Philips XL-30® scanning electron microscope (SEM). The samples were coated with a thin layer of gold to get conductivity using a sputter coater and scanned under SEM with an accelerating voltage of 10 kV. The EDX analysis of these samples was carried out using a field emission gun scanning electron microscope (FEG SEM JSM-7600F®) to determine the quantity of the elements present on the surface and cross section, and the same was expressed in atomic percentage. Bruker Innova® Atomic Force Microscope (AFM) was used to evaluate surface topography of cotton fabrics. Fibres from treated and control fabrics were removed and fixed in the stub with the help of adhesive and observed under AFM using tapping mode. Transmission electron microscope (TEM) analysis was carried out using Philips CM 200® analyser. Fibre from treated fabric was removed and kept in the TEM stub and used for the analysis after staining.
Analysis of zinc content
Zinc content analysis of treated fabrics was carried out in Atomic Absorption Spectrophotometer (GBC scientific equipment, Avanta PM, Australia). About 1 g of cotton fabric was dissolved in hot conc. nitric acid followed by the addition of conc. sulphuric acid. After cooling, the solution was diluted to 500 ml using deionized water. Control blank was also prepared without cotton fabric.
Thermal analysis
Differential scanning calorimetry (DSC) of control and nano-ZnO treated fabrics was carried out using Mettler Toledo DSC thermal analyser. The heating rate was set at 10 °C/min in the Nitrogen atmosphere. The flammability characteristic of control and nano-ZnO treated textiles was studied by igniting the fabric vertically in a stand.
XRD analysis
X-ray diffractometer (XRD) [(Ultima IV, Rigaku, Japan with Cu Kα (λ = 1.54 Å] irradiation was used to identify the crystalline phase and to determine the grain size of the synthesised nano-ZnO on cotton fabrics. Cotton fabric was cut into pieces and powdered in a grinding mill before the analysis. XRD of pure nano-ZnO purchased from Sigma Aldrich was used for comparative purpose.
Physical and low stress mechanical properties
The tactile characteristics such as tensile, bending, shear, compression, surface friction, variation in surface roughness and air resistance of control and nano-ZnO treated fabrics were evaluated using Kawabata Evaluation System (KESF). Tensile strength of cotton woven fabric was evaluated using Instron® tensile tester. Five specimens were used for the tensile test measurement of the sample. Cotton fabric specimen of 10 cm length and 2.5 cm width were used for the testing. The values of the control and treated samples were compared to study the effect of nano-ZnO treatment on the mechanical properties of the treated samples. The Owens, Wendt, Rabel and Kaelble method was used for calculating the surface free energy of cotton fabrics from the contact angles of water and diiodo methane. Whiteness index (CIE W.I.) of the cotton fabrics was evaluated using Premier Colourscan 5000 spectrophotometer.
Assessment of wash durability
Finish durability of treated cotton fabrics for UV protective and antimicrobial functional properties was evaluated after washing the fabric as per AATCC-61-2003 method (1A) using 0.37% detergent at 40 °C for 45 min. One cycle of wash is equal to five hand washes.
After each wash cycle, wash liquor was collected. This liquor was digested using hot conc. nitric acid followed by the addition of conc. sulphuric acid. After cooling, the solution was diluted to 500 ml using deionized water. Quantitative analysis to determine the amount of zinc in the solution was carried out using AAS. Necessary dilution of the solution was done wherever required.
Results and discussion
Optimization and mechanism for formation of nano-ZnO onto cotton fabric
To optimize the concentration of Zn(NO3)2·6H2O and NaOH for in situ formation of nano-ZnO on cotton fabric, experiments were carried out (Table 1). It is evident from the results that 0.25 N Zn(NO3)2·6H2O and 0.5 N NaOH concentrations are optimum in terms of the antibacterial and UPF properties of the fabric.
However, methanol emerged as a suitable treatment medium in terms of enhancing the UV protection properties of treated fabrics (Table 2). Possible reason for higher UPF in case of methanolic medium may be the entrapment of more amount of zinc hydroxide (Zn(OH)2) in cotton fabric due to its insolubility in methanolic medium which later on transformed into ZnO after heating. Solubility product (Ksp) of Zn(OH)2 in water is 3.0 × 10−16 at 25 °C, which further increases in aqueous alkaline medium causing the leaching of Zn(OH)2 as the suspension in aqueous medium. In addition, methanol may assist the conversion of zinc hydroxide into zinc oxide due to its dehydrating property. The mechanism for formation of nano-ZnO on cotton fabric is given in Eqs. 1 to 3 and schematically presented in Fig. 1.



Conventionally metal oxides were synthesised on cotton textiles to produce mineral khaki colour (Race et al. 1945). In the present work, cotton fabric is used as template to synthesize uniform size of ZnO across the fibre surface. The primary wall of cotton fibre has relatively shorter cellulosic chains than in the secondary wall and contains ultrafine pores throughout its surface (Bertoniere and King 1989). On swelling with solvent, pore size gets increased and provide path to nitrate anions and zinc cations to penetrate through and interact with the shorter cellulosic chain via hydrogen bonds. Existing intermolecular hydrogen bonds in the shorter cellulosic chain due to combined effect of anions and cations are loosened, that further increases the surface pore size of cellulosic fibres. On treatment with NaOH, Na+ and OH− approach zinc nitrate and the reaction begins inside pores to form insoluble zinc hydroxide. After the removal of solvent (methanol), cellulose molecules retain their original position resulting in the reduction in the pore size and ultimately the physical entrapment of Zn(OH)2 inside the fibre pores takes place. Zn(OH)2, a germinal diol with two OH groups on the same atom is thermodynamically unstable due to strong electrostatic repulsion between lone pair of electrons of adjacent oxygen of OH groups. The electrostatic repulsion causes the increase in the energy of the molecule and reduces its stability. On heating, water molecules are released to convert unstable Zn(OH)2 into stable ZnO.
Confirmation of formation of nano-ZnO
SEM of the treated cotton fabric (Fig. 2) clearly shows the presence of nano-ZnO particles on the fabric surface. The energy-dispersive X-ray analysis of the control, nano-ZnO treated fabric and the cross section of fibres from nano–ZnO treated fabric is reported in Table 3. As expected, the control sample showed only the presence of carbon and oxygen atoms. However, in nano-ZnO treated fabric zinc could be detected. It is also interesting to find that the cross section of fibres obtained from the nano-ZnO treated fabric also showed the presence of zinc in EDS analysis, which confirms the presence of ZnO not only in the surface of the treated fabric but also in cross section of the cotton fibre. Figure 3 presents the XRD patterns of in situ deposited ZnO nanoparticles of cotton fabric. Diffractogram of nano-ZnO embedded fabric gives the indication of crystalline nature of ZnO. The peaks at 2θ = 31.77°, 34.53°, 36.45°, are assigned to the (100), (002), and (101) planes and confirm the formation of Wurtzite phase hexagonal structure of ZnO (Khosravian et al. 2015). Using the Scherrer equation, the grain size of the nano-ZnO was found to be 35 nm. TEM analysis of nano-ZnO treated cotton fibre has been done (Fig. 4). It was found out that nano-ZnO was embedded in cotton fibrils and selected electron diffraction (SAED) pattern revealed the formation of Wurzite structure of ZnO. Figure 5 shows the FTIR spectra of control and nano-ZnO treated cotton fabrics. The peaks at 1420 and 900 cm−1 are attributed to CH2 and C–OH bending of cellulose of cotton fabric, respectively. A peak appearing at 451 cm−1 in treated fabric due to Zn–O stretching frequency (Uysala et al. 2013) confirm the formation of nano-ZnO on cotton fabric.
Antibacterial and ultra-violet protection properties
ZnO differs from other metal and metal oxide nano particles in terms of bactericidal property due to requirement of comparatively higher concentration to inhibit the growth of pathogenic microorganisms as it is an essential micro nutrient for prokaryotic organisms. However, inhibition of many bacteria by zinc ion at higher concentration (Sirelkhatim et al. 2015) illustrates that concentration of ZnO in the cotton fabric is one of the very important factors to kill pathogenic bacteria. Antibacterial property of treated cotton fabric was quantitatively evaluated against the Gram-positive bacterium Staphylococcus aureus and the Gram-negative bacterium Klebsiella pneumoniae. As shown in Table 2, control fabric did not show any inhibition against both the organisms. Whereas, nano-ZnO treated cotton fabrics exhibited excellent inhibition (100%) against S. aureus and K. pneumonia. It is reported that Gram-positive bacteria are more susceptible to inhibition by ZnO particles compared to Gram-negative bacteria and thus growth inhibition of Gram-negative bacteria occurred at higher ZnO concentrations. The inhibition accounts for variations in cell physiology, cell wall constitution and the metabolism (Atmaca et al. 1998). Results also support the differential inhibition property of nano-ZnO particles on the Gram-positive bacterium Staphylococcus aureus and the Gram-negative bacterium Klebsiella pneumoniae (Table 1). Antibacterial property of ZnO against different microorganisms has been well reported by many researchers (Vigneshwaran et al. 2006 and Perelshtein et al. 2009). The present method is simple and fast to develop durable antimicrobial cotton fabric as compared to reported in situ methods.
ZnO is a bifunctional material and besides antibacterial prosperity, it also displays UV blocking attribute due to its wider band gap energy. Existing reported methods could improve UPF marginally in nano-ZnO finished cotton fabric (Shaheen et al. 2016). In addition, little research has been done to study the UV protecting properties of nano-ZnO synthesized directly on cotton fabric. Under this work, UPF activity of nano-ZnO treated cotton fabrics was examined in details and results are given in Table 3. Control fabric exhibited very less UPF mean (5) due to its inherent nature of less thickness and aerial density, whereas, excellent improvement in the UPF of in situ synthesised nano-ZnO treated fabric (42) was obtained as compared to control fabric. Achieving UPF value above 40 indicates the better efficiency of this method to produce UV protective cotton textiles. UV absorption spectra of nano-ZnO treated cotton fabric showed that due to the presence of nano ZnO there is strong absorption of UV light in the range of 260 −370 nm and transmitting <3% of UV light while the control fabric is transmitting more amount (>15%) of UV light.
Thermal properties
The DSC analysis was carried out for both control and nano-ZnO treated cotton fabrics to determine their thermal behaviour (Fig. 6). An endothermic peak observed in control and nano-ZnO treated cotton fabrics in the range of 60–110 °C is attributed to heat of evaporation of water molecules present in the fabric. For nano-ZnO treated fabric, an endotherm observed at 125 °C may be attributed to the decomposition of small quantity of Zn(OH)2 present in the fabric. Control cotton fabric showed an endotherm in the range of 360–385 °C with the peak at 372 °C corresponding to the depolymerisation of cellulose with the formation of flammable gases like pyro glucosan and levo glucosan. At this temperature, pyrolytic degradation of cotton takes place with a rapid cleavage of the glucosidic bond (Basak et al. 2015). Unlike control fabric, nano-ZnO treated cotton fabric showed entirely different peak after 290 °C. An exotherm appeared in the range of 360–380 °C with a peak at 372 °C, which may be attributed to the crystallization process of ZnO. Moreover, pyrolytic degradation of cellulose in the treated cotton fibre has shifted to comparatively lesser temperature with no definite endothermic peak. This behaviour is attributed to catalytic degradation of cellulose in the presence of ZnO. This property of ZnO may be beneficial for the development of flame retardant textiles (El-Hady et al. 2013). The flammability characteristics of control and nano-ZnO treated textiles were observed by igniting the fabric by keeping the fabric in a vertical stand (Fig. 7). Propagation of flame time in the treated textile was comparatively lesser and more amount of char was formed while control fabric was burnt out completely as evident from the Fig. 7.
Mechanical properties
Tensile strength: Strength analysis of nano-ZnO treated cotton fabric showed 5.43% reduction in the tensile strength for the warp direction. However, no major change in the strength of treated fabric in weft direction was observed. Other researchers have also reported that incorporation of nano-ZnO on cotton fabric may marginally affect the mechanical strength (Dhandapani et al. 2014). This finding indicates that the mechanical properties of the cotton fabric loaded with nano-ZnO particles do not get much influenced.
Air resistance: The air permeability of fabrics is of paramount importance in terms of comfort property. Air permeability of cotton fabric is reduced if it is coated with any film forming polymeric materials such as binders. In the present study, no significant reduction in the air resistance of the treated fabric was observed which indicates that the interstices of yarn spaces are not blocked with nano-ZnO.
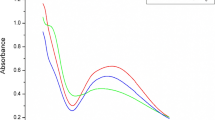
Whiteness: Whiteness index of the treated cotton fabric with respect to the control fabric was found to be reduced to 60.33 from 75.16. This could be due to yellowing of fabric during curing process and some absorption in the blue region (400–420 nm) of visible light by the treated fabric. This is evident from the UV absorption spectra of the control and treated fabrics (Fig. 8).
Surface energy: Surface energy of the control and treated textiles was calculated using Owens, Wendt, Rabel and Kaelble method by measuring the surface contact angle of two different liquids of different surface tension, i.e. water and diiodo methane (Owens and Wendt 1969). Surface free energy of the treated fabric was found to be slightly higher (76.3) than that of untreated cotton fabric (76.0). Surface energy is directly related with wetting behaviour of textiles. Higher surface energy favours the wetting of liquids. Increase in the surface energy is attributed to more roughened surface due to the impregnation of nano-ZnO on the treated surface. AFM analysis of the treated and control fabrics indicated the presence of ZnO causing roughened surface (Fig. 9). Surface area (2 µm × 2 µm) of the treated textile was increased from 6.3 to 8.2 µm2 due to of deposition of nano-ZnO, which indicates development of micro roughness on the treated textile. This was further substantiated by the increased surface friction in the treated fabrics using Kawabata surface analysis (Table 4). These results demonstrate that the liquid moisture absorption properties of the treated textiles will not be hampered due to deposition of nano-ZnO particles.
Low stress mechanical properties: The Kawabata Evaluation System for Fabrics (KESF) is used in this study for evaluating low stress mechanical properties such as tensile, shearing, bending, compression and surface properties, which are used for expressing fabric handle in apparel industries. The study of tensile properties of fabrics, including tensile energy (WT), tensile resilience (RT) and extensibility (EMT) is important because apparel usually undergoes small extension and relaxation during wear. As shown in Table 5, RT of the treated fabric was higher than control fabric, it increased by 30.3 and 15.1% in warp and weft directions, respectively. Presence of nano-ZnO on cotton fabric surface and between the fibres might have resulted in resistance to the tensile stress (Kan and Lam 2013). This supports reduced EMT % of the treated fabric in both the directions. Shear is a significant determinant of handle and drape of fabrics and a fabric with low shear rigidity (G) values indicates superior shearing properties. Average G value for treated fabric increased by 40.0% as compared to the control fabric. The rise in G value could possibly be attributed to the combined effect of roughened fabric surface and partial agglomeration of the nano-ZnO particles. The bending property of a fabric is assessed by bending rigidity (B) and low B value signifies better bending properties. In treated cotton fabric, average bending rigidity increased by 23% than control cotton fabric. Surface properties like coefficient of friction (MIU) indirectly indicates the surface roughness of the textile materials. Higher average MIU value of 0.250 was obtained for treated cotton fabric as against 0.198 of the control fabric. Higher MIU values are attributed to uneven surface resulted by the deposition/agglomeration of nano-ZnO on cotton fabric. This study shows that shear, bending and surface properties are getting affected due to the deposition of nano-ZnO on cotton fabric, which affects primary hand values of the treated fabric (Table 6). However, the difference in the total hand value of the control (3.52) and treated fabric (3.40) was marginal indicating the effectiveness of this method.
Effect of laundering on the antibacterial property and UV protection of cotton fabrics
Washing durability of textile is very important aspect for the materials finished with functional chemicals. Metal oxide particles are bound to polymeric chain of textile material by very weak forces such as Van der Waal’s, etc. Since nanomaterials are very small in size, they try to come out from the material during washing process. Washing durability of in situ generated nano-ZnO cotton fabrics was therefore, evaluated using AATCC 61 method. Results given in Table 4 show that cotton fabric treated with nano-ZnO retained its anti-bacterial and UV protective properties even after 30 washes with optimum quantity of ZnO. Leaching out of ZnO was evaluated by measuring the zinc content in the washed off liquor and results presented in Table 7. Amount of zinc leached from the fabric was initially more and reduced drastically in further wash cycles indicating that only superficially attached nano-ZnO particles are leached out. SEM images of washed fabrics still display ZnO particles on surface of the fibre. To understand the reason for very good washing durability of in situ generated nano-ZnO particles on cotton fabric, cross section of treated cotton fabric was observed under SEM wherein some ZnO particles seen embedded beneath the surface of fibre (Fig. 2). This confirms our hypothesis of ZnO formation in the pores of the cotton fibre. UV protection of the treated fabrics was also retained after laundering. In other reported methods (Shaheen et al. 2016) UPF of the treated fabrics reduced drastically just after 15 washes. This method of synthesis resulted in very good UV protection even after 30 washes.
Conclusion
We have reported a very simple and effective method for producing durable antibacterial and UV protective finish to cotton fabrics by synthesizing nano-ZnO directly on cotton fabric. The pores present on the surface of cotton fibre were targeted as reaction sites for nucleation of ZnO to ensure the uniformity for the size of ZnO particles and their physical entrapment for durability. Concentration of precursors for the in situ synthesis influences the final quantity of nano-ZnO that is loaded on cotton fabric. Nano-ZnO was deposited not only on the surface of cotton fibre but also formed inside the fibre, which was confirmed by SEM analysis. Optimum quantity of ZnO was retained by the cotton fabric after laundering that facilitated durable antibacterial and ultra-violet protective properties of the treated fabric. Most of the physical properties of cotton fabric were unaffected by this synthesis.
References
Arputharaj A, Prasad V, Saxena S, Vigneshwaran N, Shukla SR (2016) Ionic liquid mediated application of nano zinc oxide on cotton fabric for multi-functional properties. J Text Inst. doi:10.1080/00405000.2016.1222984
Atmaca S, Kadri GÜL, Ciçek R (1998) The effect of zinc on microbial growth. Turk J Med Sci 28:595–598
Baldwin S, Odio MR, Haines SL, O’Connor RJ, Englehart JS, Lane AT (2001) Skin benefits from continuous topical administration of a zinc oxide/petrolatum formulation by a novel disposable diaper. JEADV 15:5–11
Basak S, Samanta KK, Chattopadhyay SK (2015) Fire retardant property of cotton fabric treated with herbal extract. J Text Inst 106:1338–1347
Bertoniere NR, King WD (1989) Effect of scouring/bleaching, caustic mercerization, and liquid ammonia treatment on the pore structure of cotton textile fibers. Text Res J 59:114–121
Dhandapani P, Siddarth AS, Kamalasekaran S, Maruthamuthu S, Rajagopal G (2014) Bio-approach: ureolytic bacteria mediated synthesis of ZnO nanocrystals on cotton fabric and evaluation of their antibacterial properties. Carbohydr Polym 103:448–455
El-Hady MA, Farouk A, Sharaf S (2013) Flame retardancy and UV protection of cotton based fabrics using nano ZnO and polycarboxylic acids. Carbohydr Polym 92:400–406
Gokarneshan N, Rachel DA, Rajendran V, Lavanya B, Ghoshal A (2015) Emerging research trends in medical textiles. Springer, Singapore
Kan CW, Lam YL (2013) Low stress mechanical properties of plasma-treated cotton fabric subjected to zinc oxide-anti-microbial treatment. Materials 6:314–333
Kathirvelu S, D’souza L, Dhurai B (2009) UV protection finishing of textiles using ZnO nanoparticles. Indian J Fibre Text 34:267–273
Khosravian S, Montazer M, Malek RM, Harifi T (2015) In situ synthesis of nano ZnO on starch sized cotton introducing nano photo active fabric optimized with response surface methodology. Carbohydr Polym 132:126–133
Owens DK, Wendt RC (1969) Estimation of the surface free energy of polymers. J Appl Polym Sci 13:1741–1747
Perelshtein I, Applerot G, Perkas N, Wehrschetz-Sigl E, Hasmann A, Guebitz GM, Gedanken A (2009) Antibacterial properties of an in situ generated and simultaneously deposited nanocrystalline ZnO on fabrics. ACS Appl Mater Interfaces 1:361–366
Race E, Speakman JB, Rowe FM (1945) The dyeing of cotton with mineral khaki part vii-the fungicidal and bactericidal efficiencies of cotton yarn treated by various mineral khaki processes. J Soc Dyers Colour 61:310–321
Saif MJ, Zia KM, Rehman FU, Ahmad MN (2015) An eco-friendly, permanent, and non-leaching antimicrobial coating on cotton fabrics. J Text Inst 106:907–911
Shaheen TI, El-Naggar ME, Abdelgawad AM, Hebeish A (2016) Durable antibacterial and UV protections of in situ synthesized Zinc oxide nanoparticles onto cotton fabrics. Int J Biol Macromol 83:426–432
Sirelkhatim A, Mahmud S, Seeni A, Kiran S, Gulzar T (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett 7:219–242
Subash AA, Chandramouli KV, Ramachandran T, Rajendran R, Muthusamy M (2012) Preparation, characterization, and functional analysis of zinc oxide nanoparticle-coated cotton fabric for antibacterial efficacy. J Text Inst 103:298–303
Uysala I, Severcana F, Evis Z (2013) Characterization by Fourier transform infrared spectroscopy of hydroxyapatite co-doped with zinc and fluoride. Ceram Int 39:7727–7733
Vigneshwaran N, Sampath K, Kathe AA, Varadarajan PV, Prasad V (2006) Functional finishing of cotton fabric using zinc oxide—soluble starch nano composites. Nanotechnology 17:5087–5095
Acknowledgments
Authors are thankful to Dr. P. G. Patil, Director, ICAR-CIRCOT for his guidance and permission to publish this paper. The authors would like to acknowledge SAIF, IIT-Bombay for the EDX and TEM characterization.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arputharaj, A., Nadanathangam, V. & Shukla, S.R. A simple and efficient protocol to develop durable multifunctional property to cellulosic materials using in situ generated nano-ZnO. Cellulose 24, 3399–3410 (2017). https://doi.org/10.1007/s10570-017-1335-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1335-5