Abstract
Bacterial cellulose (BC) is a promising biomaterial as well as a model system useful for investigating cellulose biosynthesis. BC produced under static cultivation condition is a hydrous pellicle consisting of an interconnected network of fibrils assembled in numerous dense layers. The mechanisms responsible for this layered BC assembly remain unknown. This study used calcofluor as a fluorescent marker to examine BC layer formation at the air/liquid interface. Layers are found to move downward into the media after formation while new layers continue to form at the air/liquid interface. Calcoflour is also known to reduce the crystallinity of cellulose, changing the mechanical properties of the formed BC microfibrils. Consecutive addition and accumulation of calcofluor in the culture medium is found to disrupt the layered assembly of BC. BC crystalllinity decreased by 22 % in the presence of 12 % calcofluor (v/v) in the medium as compared to BC produced without calcofluor. This result suggests that cellulose crystallinity and the mechanical properties which crystallinity provides to cellulose are major factors influencing the layered BC structure formed during biosynthesis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacterial cellulose (BC), biosynthesized by Gluconacetobacter xylinus, is a highly pure cellulose hydrogel (Iguchi et al. 2000). Similarities in the chemical structure and biosynthesis of plant and bacterial cellulose have generated interest in the use of BC as a model system to study the formation and organization of cellulose in the plant cell wall (Mikkelsen et al. 2009). The mechanism behind the assembly of BC fibrils has been proposed in detail (Valla et al. 2009). It involves the assembly of subfibrils which further assemble into microfibrils and then ribbons of cellulose. Subfibrils are about 1.5 nm wide consisting of 3–4 cellulose glucan chains synthesized by cellulose synthase enzymes. These enzymes, which exist in the form of a multi-enzyme complex, are located in the outer membrane of the bacterial cell wall of many cellulose producing strains including G. xylinus. Numerous subfibrils are extruded from bacterial cells into the medium where they are crystallized into microfibrils and ribbons through the formation of inter-chain hydrogen bonds (Bielecki et al. 2005; Jonas and Farah 1998; Yamanaka et al. 2000). The macro-structural assembly of BC is strongly influenced if not controlled by the micro-assembly of extruded BC ribbons. Under static cultivation condition, BC exhibits a sheet-like morphology with relatively dense BC layers connected by BC ribbons or microfibrils (Hu et al. 2014). Under a series of orbital shaking cultivation conditions, some bacterial strains can produce BC in the form of sphere-like shapes consisting of an irregular fibrous structure in the center surrounded by loose BC layers (Hu and Catchmark 2010a; Hu et al. 2013).
Calcofluor is a fluorescent whitening agent for cellulose that can compete with water molecules to chemically bind to cellulosic macromolecules (Haigler et al. 1980). The presence of calcofluor in the BC growth medium can impact the crystallization of BC fibrils and disrupt the micro-assembly of BC fibrils. Calcofluor can also function as a cell poison to inhibit cell production and high concentrations of calcofluor in the medium can lead to a reduction of BC yield (Colvin and Witter 1983). Therefore, the relationship between the calcofluor concentration and the sheet-like shape of BC pellicles requires additional investigation. Many extraneous factors can impact the micro-assembly of BC fibrils. For example, the enzymatic degradation of cellulose could cause an irreversible collapse of cellulose structure (Hu and Catchmark 2011a, b). Some factors may not directly affect the ultimate shape of a BC pellicle, but may impact the micro-assembly of BC fibrils resulting in different changes of BC properties. One example is the addition of nalidixic acid and chloramphenicol to the BC culture medium resulting in the elongation of bacterial cells which produced wider cellulose ribbons and BC pellicles with enhanced mechanical properties (Yamanaka et al. 2000). Although the effect of calcofluor on the crystallization of BC has been well studied, the impact of additives that disrupt cellulose crystallization on the assembly of BC fibrils, layers and pellicles has not been explored. In this work, the impact of calcofluor on the pellicle assembly and formation is investigated. It is found that reduced cellulose crystallinity results in a disruption of BC pellicle layer formation. It is hypothesized that this occurs due to a reduction in the stiffness of cellulose microfibrils and ribbons, which is crucial to layer formation. A model is proposed explaining layer formation in statically cultivated BC. Moreover, conclusive evidence is presented on the direction of BC pellicle growth, in agreement with previous studies.
Materials and methods
Materials
Cellulose-producing bacteria, Gluconacetobacter xylinus JCM 9730 strain (ATCC 700178) were purchased from American Type Culture Collection. Calcofluor (Fluka 18909) was purchased from Sigma-Aldrich (USA). Calcofluor is a cellulose binding fluorochrome (Colvin and Witter 1983). Prior to use, calcofluor was filtered using 0.22 μm filter to ensure sterility.
Preparation of initial bacterial inoculum
The initial bacterial inoculum was prepared using our previous protocol (Hu and Catchmark 2010b). G. xylinus JCM 9730 strain was dissolved in 1 L pH 5.0 nutrient medium consisting of 20.0 g glucose, 5.0 g yeast extract, 5.0 g bacterial peptone, 2.7 g sodium phosphate dibasic, 1.2 g citric acid, 1.0 g magnesium sulfate, 2.0 g ammonium sulfate and 1 mL corn steep supernatant. A three-day static incubation at 30 °C was utilized. One percent cellulase (v:v) sterilized with 0.22 μm filter (Sigma C2730) was added to the medium and hydrolyzed the synthesized cellulose at 37 °C until no visible pellicle was shown. Next, the mixture was centrifuged at 10,000 rpm at 4 °C to separate bacterial cells from the mixture. The pellet of bacterial cells was collected and rinsed with fresh medium by repeating the centrifugation. At the end, the fresh medium was added to the cell pellet to make the bacterial cell inoculum.
BC cultivation with different concentrations of calcofluor
Different concentrations of calcofluor were added to the culture media according to Table 1. Each sample containing 100 mL of medium was inoculated with 1 mL of the initial bacterial inoculum. The ‘One-time addition’ experiment involved the addition of 1 mL calcofluor to the medium at the assigned culture time: 6th, 12th, 24th, 36th, 48th h, and the total volume of calcofluor was 1 mL in this case. ‘Consecutive addition’ was to add 1 or 3 mL calcofluor to the medium every 12 h until a 48 h culture time was reached, and the total volume of calcofluor was 4 and 12 mL, respectively, for these cases. The one-time addition of calcofluor was designed to investigate the impact of calcofluor at a fixed concentration at different culture times. Consecutive addition was designed to evaluate the impact of calcofluor at an increasing concentration gradient. All samples were incubated at 30 °C for 72 h under the static culture. Photographic observation was recorded using a digital camera.
FESEM and XRD analysis
Harvested BC pellicles were lyophilized and imaged by FESEM (LEO 1530, Germany) operating at 1 kV. Samples were prepared by the protocols described in the reference (Ruan et al. 2014a, b). All samples were coated with Platinum using a vacuum sputter coater to improve sample conductivity.
XRD patterns of the samples were collected on a PANalytical X’Pert Pro MPD θ/θ goniometer (Almelo, the Netherlands) with Cu-Kα radiation, and fixed slit incidence (0.5° divergence, 1.0° anti-scatter, specimen length 10 mm) and diffracted (0.5° anti-scatter, 20 µm nickel filter) optics. Scans were collected at 2° per minute from 5° to 40° 2θ. Prior to XRD scans, the T-Rex system (TRX-1000-D) was used to press the lyophilized BC samples to ensure an identical size and thickness. The compression was performed under 10 MPa pressure for 2 min and the resulting samples then were cut to uniform sheets (2.5 × 2.5 cm). Segal crystallinity index has been used to calculate the crystallinity of cellulose (French and Cintrόn 2013). However, due to possible peak overlap in the amorphous area and less accuracy for cellulose II, PeakFit software (www.sigmaplot.com) was used to perform peak deconvolution and evaluate the crystallinity index in this study. The crystallinity of samples was calculated by dividing the total peak area of all the crystalline peaks at around 14.6°, 16.9°, 22.7° and 34.5° by the total peak area of all crystalline peaks plus amorphous peak at around 21° according to the Eq. (1) described in our previous study (Hu et al. 2016; Park et al. 2010). The crystal size was calculated according to the corrected Scherrer Eq. (2) (Gu et al. 2013):
where \(\varSigma A_{cryl}\) is the integrated area of all crystalline peaks and \(\varSigma A_{amph}\) is the integrated area of all amorphous peaks; \(B_{hkl}\) is the average crystal width of a given plane (\(hkl\)); \(K\) is a constant of 0.9; \(\lambda\) is the wavelength of the incident X-rays with regards to the XRD instrument (λ = 0.1542 nm); \(\theta\) is the center angle (in degrees) of the specific peak; \(\Delta 2\theta\) (in radians) is the full width at half maximum (FWHM) of the corresponding peak and can be read from the fitting results; and \(\Delta 2\theta_{inst}\) is the instrumental broadening (0.0018 radians).
Crystal allomorphs (cellulose Iα and Iβ) were analyzed by the equation (\(Z = 1693d_{1} - 902d_{2} - 549\)) developed by Wada and co-workers (Wada et al. 2001), where \(d_{1}\) is the d-spacing of 010 (\(hkl\)) plane and \(d_{2}\) is the d-spacing of 100 plane that can be calculated from Bragg’s law. \(Z > 0\) is for the Iα-rich type and \(Z < 0\) is for the Iβ-rich type. Iα and Iβ content in the cellulose sample was calculated from two equations (Iα \(= 56.32d_{1} - 63.37d_{2}\); Iβ content \(= 64.53d_{2} - 55.69d_{1}\)) derived from the description by Wada and co-workers as well (Wada et al. 2001).
Investigation of BC film direction of assembly
Calcofluor (1 mL) was added to the BC growth medium at the beginning of the culture. The cultivation of BC pellicles was conducted according to the protocol described above. On the 5th day, BC with and without calcofluor were harvested from the culture medium. Two BC samples were rinsed with DI water to remove impurities on the pellicles, and then cut to obtain approximately 200 μm cross-section samples. The cross-section samples were placed on the glass slides and visualized using upright fluorescence microscope (Olympus BX53, Japan) and chemiluminescent imaging system (Bio-Rad ChemiDoc XRS+, USA) under UV illumination.
Results and discussions
Effect of calcofluor on sheet-like macro-assembly of BC pellicles
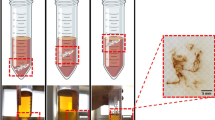
BC pellicles consist of a series of layered films composed of aggregated BC fibrils extruded from the cell membrane of G. xylinus. The addition of calcofluor to the BC culture medium was able to disrupt the crystallization of BC fibrils (Haigler et al. 1980). It also impacts the sheet-like layered assembly of BC pellicles. Figures 1 and 2 depict photographs of forming BC pellicles showing how the incorporation of calcofluor into the cultivation media is able to disrupt pellicle formation. Figure 1 shows pellicles under the influence of the one-time addition of calcofluor at different culture times, where the earlier addition of calcofluor results in a more significant impact on the sheet-like shape of BC pellicles. More disorganized fibrous BC materials were present in the medium with calcofluor added at 6th h culture time (Fig. 1b) while a relatviely thicker sheet-like BC pellicle was observed in the medium with caclofluor added at 48th h culture time (Fig. 1f). The later addition of calcofluor only binds to the nascent BC films and those already-formed BC films would not be disrupted. This suggests that the disruption by calcofluor of the macro-assembly of BC pellicles only occurrs during the BC biosysthesis. Once the sheet-like BC pellicles are formed, calcofluor may not compete with cellulose–cellulose interactions to disrupt the sheet-like shape. Figure 2 shows the consecutive addition of calcofluor at different culture times, which further confirms that calcofluor only disrupts the sheet-like macro-assembly of BC pellicles during formation. Although the consecutive addition of calcofluor results in an accumulation of calcofluor and a persistent effect on the sheet-like BC pellicle, those BC films formed before the addition of calcofluor were still present in the medium. It was noticed that the thickness of BC pellicles at either 1 or 3 mL calcofluor concentrations could continue to increase, suggesting that the calcofluor was completely bound by nascent BC films within 12 h. Higher concentrations of calcofluor (12 mL) may lead to several patchy and irregular BC pellicles (Fig. 2b4–b5) as compared to the thin but intact BC pellicle of about 1 mm (Fig. 2a4). As shown in Fig. 2c, the thickness of BC pellicles at 72nd h culture time is about 3–5 mm in the normal culture medium without calcofluor (Hu et al. 2013). It is likley that adding higher concentrations calcofluor would ultimately destroy completely the sheet-like macro-assembly of BC pellicles.
Macroscopic observation of the sheet-like macro-assembly of BC pellicles synthesized at 72nd h culture time a in the absence of calcofluor and with one-time addition of 1 mL calcofluor at the assigned culture time: b 6th h; c 12th h; d 24th h; e 36th h; f 48th h. On each photo two red arrows indicate the formed pellicle and one back arrow indicates the random cellulose fibers shown at the bottom of flask
Macroscopic observation of the sheet-like macro-assembly of BC pellicles synthesized at 24th, 36th, 48th and 72nd h culture time with consecutive addition of a1–a4 1 mL calcofluor and b1–b4 3 mL calcofluor at 12th, 24th, 36th and 48th h; c BC pellicle without calcofluor harvested at 72nd h and b5 BC pellicle in the presence of 12 mL calcofluor harvested at 72nd h. Red arrows indicate the formed pellicles
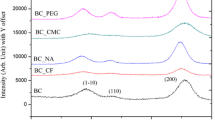
FESEM images in Fig. 3 show the micro-assembly of BC pellicles with or without the addition of calcofluor. Under static culture, BC could form a sheet-like pellicle consisting of a series of layered BC films with a distance of 5–10 µm between layers (Fig. 3a, b). The addition of 12 mL calcofluor significantly disrupted such a layered micro-assembly of BC films as shown in Fig. 3c, d where an irregular micro-assembly of BC fibrils was shown in the cross-sectional FESEM images of BC pellicles. XRD patterns of BC pellicles harvested from the medium with or without calcofluor are shown in Fig. 4 and crystallinity data are summarized in Table 2. All the original XRD patterns were analyzed using the peak deconvolution method where a Gaussian peak shape was assumed. All XRD diffractograms indicate that the material was cellulose I. Four typical peaks representing the \(hkl\) planes of \(010, 100, 110, {\text{and}}\bar{1}\bar{1}4\) were listed and other small peaks were neglected since these four peaks represent the typical character of cellulose I (French 2014). The crystallinity of BC pellicles has some significant differences amongst various samples while crystal sizes on different \(hkl\) planes remain unchanged. Z values in Table 3 indicate that all the BC samples are Iα-rich type and Iα content remains unchanged as well for all the BC samples regardless of the involvement of calcofluor in the medium. This suggests that the calcofluor only resulted in the variation of crystallinity of formed BC pellicles while it would not impact the type of crystal structure. The presence of calcofluor in the culture medium dramatically reduces the crystallinity of BC pellicles. In particular, the earlier addition of calcofluor at 6th h to the medium shows lower crystallinity than the later addition of calcofluor at 48th h. The largest loading of calcofluor (12 mL) results in a significant reduction of crystallinity from 74.71 to 58.65 %. The crystalline structure of BC appears to be a key in the formation of the sheet-like structure of BC pellicles. It is known that the crystallinity of cellulose imparts stiffness to fibrils and ribbons. This was demonstrated uniquely by observing the motion of G. xylinus bacteria on a surface where the motive force was the polymerization of cellulose in a crystalline form (Brown et al. 1976). The stiffness of the crystalline cellulose ribbon was able to translate the force of polymerization into a motive force for the bacterial cell. When calcoflour was added to the media, resulting in less crystalline cellulose, the motion ceased. It is hypothesized that the crystallinity of cellulose, which imparts stiffness to cellulose ribbons, is important to layer formation in BC pellicles. This is discussed in a proposed model of BC layer formation in section “A model of BC pellicle formation” below.
XRD patterns of BC pellicles harvested from the culture medium: (a1) without calcofluor; (a2) adding 1 mL calcolfuor at 6th h; (a3) adding 1 mL calcolfuor at 12th h; (a4) adding 1 mL calcolfuor at 24th h; (a5) adding 1 mL calcolfuor at 36th h; (a6) adding 1 mL calcolfuor at 48th h; (a7) adding 4 mL calcofluor in total; (a8) adding 12 mL calcofluor in total; b a sample showing peak deconvolution by means of PeakFit software and the parameters are set as baseline model (linear, progressive, Tol % 5.0), peak type (spectroscopy and Guassian Amplitude) and the amplitude (10 %)
Direction of assembly of BC films
Studies have shown that the macro-assembly of BC fibrils under static cultivation conditions results in a hydrous pellicle consisting of dense BC layers composed of numerous fibrils secreted from G. xylinus (Hu et al. 2016). The direction of formation of BC films is of vital importance to understand the uptake of air and nutrients by BC producing bacteria in the medium, impacting the yield of BC produced in a static culture. The literature contains limited scientific evidence regarding the direction of formation of BC films. One report described the use of a black hair as a marker attached to the initial BC films formed under static culture and found that the position of the hair moved from the air/liquid interface downward into the medium as the BC pellicle thickened during the fermentation period (Borzani and Souza 1995). Similar behavior was reported using a colored string as a noticeable marker attached to the initial BC film. The BC pellicle formed through consecutive growth of BC films at the air/liquid interface with nascent films grown on the initial BC film (Klemm et al. 2001). None of these studies provide conclusive evidence of the direction of BC film formation because neither the black hair nor colored string was chemically anchored to the initial BC film. The effect of exogenous factors may readily result in the detachment of markers or the inhibition of the organization of BC films. Therefore, a fluorescent marker, calcofluor, was applied in this study to seek conclusive evidence of the direction of formation of BC films.
Calcofluor added to the culture media can be completely consumed by binding to cellulose during the synthesis of BC films and nascent BC films will not be affected as long as the calcofluor is exhausted. Therefore, the addition of a small amount of calcofluor to the culture medium on the onset of cultivation will only enable calcofluor to be chemically anchored to the first several layers of films rather than all the other films formed after the calcofluor is exhausted. This provides a means to investigate the direction of assembly of BC films in the pellicle using calcofluor as a marker. Figure 5 shows the result measured by fluorescence microscopy. The fluorescent intensity of calcofluor bound to BC appeared to gradually increase in vertical section of BC films from bottom to top. Figure 6 depicts the result similar to Fig. 5 measured by a chemiluminescence imaging system. As compared to BC without calcofluor, a white bright band was present on the bottom of BC pellicle in the presence of calcofluor, suggesting that calcofluor was chemically anchored to the initial BC films synthesized at the air/liquid interface, and subsequently calcofluor bound BC films moved downward into the medium. The next nascent BC film was always synthesized on the previously synthesized film.
A model of BC pellicle formation
Based on an understanding of the direction of assembly of BC films, and the role of crystallinity on layer formation, a model describing the micro-assembly of layered BC is proposed and shown schematically in Fig. 7. At the beginning of BC biosynthesis, bacterial cells in the inoculum accumulate at the air/liquid interface of the culture medium due to the abundant oxygen and begin to form the first BC layer. When the interface is saturated with both cells and cellulose, a dense first layer is formed. Importantly, BC crystals are known to align to the plane of the forming pellicle. Rotating angle XRD analysis has shown that the 110 cellulose plane is oriented parallel to the air/liquid interafce (Fang and Catchmark 2014). This may result from the increased hydrophobicity of the 110 cellulose plane, and aid in confining cellulose to the interface and forming the dense layer. Once the air/liquid interface is saturated, reproduced daughter cells separate from the dense first layer as they produce cellulose. These new daughter cells, ancheored vertically at the air/liquid interface, continue to produce cellulose. The force of polymerization of this cellulose drives the first layer of cellulose down into the media, and these daughter cells begin to form the second BC layer (Tomita and Kondo 2009). These interconnecting ribbons of cellulose are seen between the dense layers of cellulose (Fig. 3b) but not seen in BC samples grown in media contianing calcofluor (Fig. 3d). The downward movement of the first BC layer is also probably aided by gravity. This process continues until the exhaustion of culture nutrients. It is further hypothesized that the layer separation is comparable to the cellulose ribbon polymerization rate multiplied by the average cell division time.
Our previous study showed that the top layer of BC films in the pellicle is denser than the bottom layer although they both have a denser fibrous assemby in the BC film than middle layers in the BC pellicle (Hu et al. 2014). This suggests that the amount of cells on the air/liquid interface is much more abundant than the deep medium and the oxygen diffusion is contrainted by the denser top layer of BC films. Therefore, cells on the first layer of BC films that has already moved downward did not have the same sythentic competency of BC fibrils as the cells were prone to float on the air/liquid interface due to the aboudant oxygen. Both denser top and botton layers of BC films in the pellicle formed the oxygen and nutrient barriers to limit the diffusion of oxygen and nutrients, which led to the loose layers of BC films in the middle of BC pellicle.
Conclusions
This work examines the impact of cellulose crystallinity on layer formation in statically cultivated BC using calcofluor as an agent to disrupt cellulose crystallinity. FESEM data showed that the layered micro-assembly of BC films in the pellicle was disrupted by the addition of calcofluor to the culture medium, which also prevented the formation of sheet-like BC pellicles. These results suggest that the crystalline structure of BC, or more importantly, the mechanical stiffness of cellulose ribbons, is important to the formation of layered BC pellicles. A model is presented explaining the process of BC layer formation. Such insights may be important to designing new processes for the microbial production of advanced microstructured biocomposites.
References
Bielecki S, Krystynowicz A, Turkiewicz M, Kalinowska H (2005) Bacterial cellulose. Biopolym Online. doi:10.1002/3527600035.bpol5003
Borzani W, Souza SJ (1995) Mechanism of the film thickness increasing during the bacterial production of cellulose on non-agitated liquid media. Biotechnol Lett 17:1271–1272. doi:10.1007/BF00128400
Brown MR, Willison JH, Richardson CL (1976) Cellulose biosynthesis in Acetobacter xylinum: visualization of the site of synthesis and direct measurement of the in vivo process. Proc Natl Acad Sci USA 73:4565–4569
Colvin RJ, Witter DE (1983) Congo red and calcofluor white inhibition of Acetobacter xylinum cell growth and of bacterial cellulose microfibril formation: isolation and properties of a transient, extracellular glucan related to cellulose. Protoplasma 116:34–40. doi:10.1007/BF01294228
Fang L, Catchmark JM (2014) Characterization of water-soluble exopolysaccharides from gluconacetobacter xylinus and their impacts on bacterial cellulose crystallization and ribbon assembly. Cellulose 21:3965–3978. doi:10.1007/s10570-014-0443-8
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896. doi:10.1007/s10570-013-0030-4
French DA, Cintrόn MS (2013) Cellulose polymorphy, crystallite size, and the Segal crystallinity index. Cellulose 20:583–588. doi:10.1007/s10570-012-9833-y
Gu J, Catchmark JM, Kaiser EQ, Archibald DD (2013) Quantification of cellulose nanowhiskers sulfate esterification levels. Carbohydr Polym 92:1809–1816. doi:10.1016/j.carbpol.2012.10.078
Haigler CH, Brown RM, Benziman M (1980) Calcofluor white ST alters the in vivo assembly of cellulose microfibrils. Science 210:903–906. doi:10.1126/science.7434003
Hu Y, Catchmark JM (2010a) Formation and characterization of spherelike bacterial cellulose particles produced by Acetobacter xylinum JCM 9730 strain. Biomacromolecules 11:1727–1734. doi:10.1021/bm100060v
Hu Y, Catchmark JM (2010b) Influence of 1-methylcyclopropene (1-MCP) on the production of bacterial cellulose biosynthesized by Acetobacter xylinum under the agitated culture. Lett Appl Microbiol 51:109–113. doi:10.1111/j.1472-765X.2010.02866.x
Hu Y, Catchmark JM (2011a) Integration of cellulases into bacterial cellulose: toward bioabsorbable cellulose composites. J Biomed Mater Res B 97B:114–123. doi:10.1002/jbm.b.31792
Hu Y, Catchmark JM (2011b) In vitro biodegradability and mechanical properties of bioabsorbable bacterial cellulose incorporating cellulases. Acta Biomater 7:2835–2845. doi:10.1016/j.actbio.2011.03.028
Hu Y, Catchmark JM, Vogler EA (2013) Factors impacting the formation of sphere-like bacterial cellulose particles and their biocompatibility for human osteoblast growth. Biomacromolecules 14:3444–3452. doi:10.1021/bm400744a
Hu Y, Catchmark JM, Zhu Y, Abidi N, Zhou X, Wang J, Liang N (2014) Engineering of porous bacterial cellulose toward human fibroblasts ingrowth for tissue engineering. J Mater Res 29:2682–2693. doi:10.1557/jmr.2014.315
Hu Y, Zhu Y, Zhou X, Ruan C, Pan H, Catchmark JM (2016) Bioabsorbable cellulose composites prepared by an improved mineral-binding process for bone defect repair. J Mater Chem B 4:1235–1246. doi:10.1039/C5TB02091C
Iguchi M, Yamanaka S, Budhiono A (2000) Bacterial cellulose—a masterpiece of nature’s arts. J Mater Sci 35:261–270. doi:10.1023/A:1004775229149
Jonas R, Farah LF (1998) Production and application of microbial cellulose. Polym Degrad Stab 59:101–106. doi:10.1016/S0141-3910(97)00197-3
Klemm D, Schumann D, Udhardt U, Marsch S (2001) Bacterial synthesized cellulose—artificial blood vessels for microsurgery. Prog Polym Sci 26:1561–1603. doi:10.1016/S0079-6700(01)00021-1
Mikkelsen D, Flanagan BM, Dykes GA, Gidley MJ (2009) Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J Appl Microbiol 107:576–583. doi:10.1111/j.1365-2672.2009.04226.x
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:1–10. doi:10.1186/1754-6834-3-10
Ruan C, Hu N, Hu Y, Jiang L, Cai Q, Wang H, Pan H, Lu WW, Wang Y (2014a) Piperazine-based polyurethane-ureas with controllable degradation as potential bone scaffolds. Polymer 55:1020–1027. doi:10.1016/j.polymer.2014.01.011
Ruan C, Hu Y, Jiang L, Cai Q, Pan H, Wang H (2014b) Tunable degradation of piperazine-based polyurethane ureas. J Appl Polym Sci 131:40527. doi:10.1002/app.40527
Tomita Y, Kondo T (2009) Influential factors to enhance the moving rate of Acetobacter xylinum due to its nanofiber secretion on oriented templates. Carbohydr Polym 77:754–759. doi:10.1016/j.carbpol.2009.02.022
Valla S, Ertesvåg H, Tonouchi N, Fjærvik E (2009) Bacterial cellulose production: biosynthesis and applications. Microbial production of biopolymers and polymer precursors: applications and perspectives. Caister Academic Press, Norfolk, pp 43–77
Wada M, Okano T, Sugiyama J (2001) Allomorphs of native crystalline cellulose I evaluated by two equatorial d-spacings. J Wood Sci 47:124–128. doi:10.1007/BF00780560
Yamanaka S, Ishihara M, Sugiyama J (2000) Structural modification of bacterial cellulose. Cellulose 7:213–225. doi:10.1023/A:1009208022957
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31570967 and 31370978); the Guangdong Province Science and Technology Planning Project (2015A010105021); the Shenzhen Science and Technology Program (JCYJ20140610152828698 and CXZZ20140417113430716); the Shenzhen Peacock Program (110811003586331).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Changshun Ruan and Yongjun Zhu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ruan, C., Zhu, Y., Zhou, X. et al. Effect of cellulose crystallinity on bacterial cellulose assembly. Cellulose 23, 3417–3427 (2016). https://doi.org/10.1007/s10570-016-1065-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-1065-0