Abstract
In this study, a novel method including the concurrent use of nanoparticles and non-solvent is proposed for surface modification of filter papers with the aim of achieving superhydrophobicity that can be used in oil/water separation applications. The effects of polymer, nanoparticle and non-solvent contents were investigated on the surface morphology, composition and wettability of the samples. A comparison was also made between the solution casting and dip-coating techniques. It was found that the dip-coating technique was not as efficient as solution casting because of the lower adsorption of polystyrene macromolecules onto the papers’ surfaces. Scanning electron microscopy results demonstrated the role of non-solvent in the surface morphology of the coatings. In fact, in the absence of non-solvent, superhydrophobicity was not achieved. X-ray photoelectron spectroscopy results confirmed that the presence of non-solvent also changed the surface composition of the coatings significantly. The optimum composition was found to have promising potentials in the separation of oil from water because of the induced superhydrophobic and superoleophilic properties. The findings of this fundamental research could be exploited in other systems for more efficient oil/water separating devices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surface modification of paper substrates has been increasingly studied with the aim of extending their applicability in novel materials including microelectronics (Siegel et al. 2009), intelligent paper actuators (Shen et al. 2010), and superhydrophobic papers for oil/water separation (Du et al. 2014). In this regard, various methods have been utilized such as photo-attaching functional polymers to cellulose fibers (Böhm et al. 2013), nanoparticle coating via the liquid flame spray technique (Teisala et al. 2014) and modification by polyvinylsilsesquioxane via solution immersion (Chen et al. 2016). One of the recent applications of filter paper modification has been the separation of oil–water mixtures, which is becoming an important worldwide concern because of the increased amounts of industrial oily wastewater and polluted oceanic water as well as oil leakage accidents (Feng et al. 2004; Yuan et al. 2008; Xue et al. 2014). Thus, many studies have been conducted within the recent years in order to develop cost-effective functional materials with the capability of oil–water separation. In fact, a diverse range of materials have been used for the development of oil-absorbing systems such as porous metal foams (Zhang et al. 2015), ultra-light carbon aerogels obtained from nanocellulose (Meng et al. 2015), porous polymer membranes (Li et al. 2016) and so on. In many of the reported systems for oil–water separation, both superhydrophobicity and superoleophilicity have been imparted to the used materials (Zhang et al. 2015; Li et al. 2016). Very few studies have also reported on separating oil/water mixtures through hydrophilic/oleophobic systems (Tang et al. 2015). In their work, wetting was tuned by paper surface modification using a thin film of hydrolyzed methyltrimethoxysilane.
Within the last 2 decades, the development of materials with special wettabilities has been the focus of numerous studies worldwide; they have a vast range of application in different industries such as the marine industry, biomedical devices and so on (Hsu et al. 2011; Seyfi et al. 2015b, 2016; Hejazi et al. 2015b, 2016). In this respect, different methods have been suggested to produce superhydrophobic materials including nanoparticle surface embedding (Hejazi et al. 2015a), electrospinning (Thorvaldsson et al. 2012), dip-coating (Zangi et al. 2016) and so on. Researchers used a combination of superhydrophobic and superoleophilic properties with the aim of developing oil–water separation systems. Several papers have reported the efficiency of filter paper as a separating unit upon surface modification (Wang et al. 2010; Arbatan et al. 2012; Zhang et al. 2012; Ogihara et al. 2013; Du et al. 2014; Gao et al. 2015). For instance, Du et al. (2014) reported the fabrication of superhydrophobic and superoleophilic filter paper for oil–water separation via a colloidal deposition method based on polytetrafluoroethylene nanoparticles. Arbatan et al. (2012) attained superhydrophobic paper via a two-step dip-coating method. In their work, precipitated calcium carbonate (PCC) was used to create the roughness, and cellulose nanofibers were used as a binder to form and retain the PCC clusters on the surface of fibers. In another work, superhydrophobic paper was produced through spraying nanoparticle suspensions (Ogihara et al. 2013), and it was found that the required concentration of nanoparticles in the suspension should be 20 g L−1, which is rather high. In a similar work to the current study, Zhang et al. (2012) reported successful fabrication of superhydrophobic filter paper via a simple solution casting technique. However, they used a very high inclusion of silica nanoparticles (50 wt% with respect to the solid content). According to the reported literature, it is realized that either the used system was expensive or the concentration of nanoparticles was exceedingly high. In this work, a novel method is presented to render the filter paper superhydrophobic and superoleophilic with the aim of oil–water separation. The proposed method is facile and needs no further modification step; also, it requires a significantly lower content of nanoparticles for achieving superhydrophobicity, which makes it more desirable for large-scale production. Moreover, non-fluorinated materials such as polystyrene and silica were employed, which make it more environmentally desirable. An attempt was also made to compare the efficiency of the drop-coating and dip-coating methods in attaining superhydrophobicity for the filter paper.
Materials and methods
Materials
Polystyrene (M w = 192,000 g/mol, softening point = 107 °C) was obtained from Sigma-Aldrich (St. Louis, MO, USA). The hydrophobic fumed silica is a commercial product (Aerosil R805) that was provided by Evonic Industries (Essen, Germany) and used as received. Aerosil R805 has a specific surface area of 150 ± 25 m2 g−1 and primary particle size of 12 nm. Tetrahydrofuran (THF) and ethanol were supplied from Merck (Darmstadt, Germany) and used as received.
Modification process
Prior to the modification process, filter papers (Whatman) were first washed with distilled water and acetone and then dried at 80 °C and 1 atm for 2 h to remove any contaminations. After that, a certain amount of PS granules (1 and 5 wt%) was dissolved in 10 ml THF followed by the addition of silica nanoparticles into the PS solution. The prepared suspension was vigorously dispersed via the magnetic stirring and ultrasonic bath methods. After attaining a homogeneous mixture, non-solvent was added drop-wise into the PS suspension. The majority of samples in this work were fabricated through the solution casting technique. To this end, several drops of the prepared suspension were cast onto the surface of filter papers that were then left to dry at ambient conditions. A few samples were also prepared via dip-coating technique in order to compare the efficiency of these two methods. Therefore, after preparation of the above-mentioned mixtures, the filter papers were dipped into the mixture for 30 min followed by drying at ambient conditions. Table 1 shows a list of prepared samples along with their formulations.
Characterizations
A video-based contact angle measurement system (OCA 15, DataPhysics Instruments GmbH, Filderstadt, Germany) was used to determine the WCA values. The WCA measurements of each sample were conducted at least three times across the sample surface using the sessile drop method by dispensing 4-µl drops of de-ionized water on the surfaces. Morphologies of the coatings’ surfaces were investigated on a digital scanning electron microscope (KYKY-EM3200, KYKY Technology Development, Beijing, China) operated at 25 kV. To avoid any electric charging, all the samples were plated with gold coating. X-ray photoelectron spectroscopy (XPS) analysis was conducted using XPS spectroscopy with a monochromatic AlKα X-ray source (1486.6 eV photons), operated at 180 W (12 kV and 15 mA) and under ultra-high vacuum conditions.
Results and discussion
Wettability
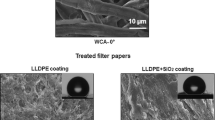
In order to measure the wetting results for the modified filter papers, water contact angle measurement was employed. The water contact angle (WCA) results are reported in Table 1, and the water droplet profiles are shown in Fig. 1 for the modified filter papers. According to Table 1, the optimum sample is P1S0.5E15, which exhibits the highest WCA (165°). Upon increasing the nanosilica content to from 0.5 to 1 wt%, not only was the WCA not increased, but it also was reduced from 165 to 158°. On the other hand, the reduction in the amount of nanosilica from 0.5 to 0.1 wt% caused a decrease in the WCA value from 165 to 155°. It is concluded that the optimum extent of nanosilica should be tuned to around 0.5 wt% with respect to the whole system. The effect of non-solvent content was also evaluated by preparation of P1S0.5E25. It was found that the increased amount of non-solvent had an adverse effect on the WCA value (151°); however, this sample still remained superhydrophobic. It should be noted that the lower content of ethanol did not result in superhydrophobicity; thus, the amount of non-solvent in this formulation should be adjusted at 15 wt%. According to the wettability results, non-solvent plays a vital role in achieving superhydrophobicity since the absence of non-solvent in the optimum formulation resulted in a significant loss in the WCA value from 165 to 112° in the case of P1S0.5. The PS concentration was also varied, and it was found that a very high content of PS in the optimum formulation (P5S0.5E15) causes a substantial decline to 101°, which is very close to the WCA value of the pure PS coating.
A very important observation was that the attained superhydrophobicity was not stable at longer periods of time since the water droplet was found to gradually wet the filter paper after 10 min. This behavior was observed for all the samples with the WCAs beyond 150° except P1S0.5E15 and P1S1E15 for which the superhydrophobic property was utterly stable till the droplet was finally evaporated. Based on this finding, it can be inferred that P1S0.1E15, P1S0.5E25 and P1S0.5E15_Dip, all of which have WCAs above 150°, should not be regarded as superhydrophobic because of their water-absorbing property at longer periods of time. Therefore, they cannot be used for the separation of oil from water since the filter papers are in continual contact with water by which they could be gradually wetted. For this reason, the optimum sample was only used for oil/water experiments. Apart from the quantified results, the water profiles during WCA measurement are also shown in Fig. 1 for different samples. The changes in water profiles are marked in this figure upon variations in non-solvent, nanoparticles and polymer contents. The surface of filter paper that has been modified with the optimum formulation is also illustrated in Fig. 1f.
Surface morphology
Scanning electron microscopy (SEM) was employed to explore the possible reasons for the observed wetting behaviors. Figure 2 shows the surface morphologies of samples made from different contents of nanosilica. It can be evidently observed that upon increasing the nanoparticle content, they became more packed and aggregated on the surface of filter paper, which is one of the reasons for the improved hydrophobicity. It is seen that micron-scale spheres were more profoundly present on the surface of P1S0.1E15, which has the lowest amount of nanosilica. Those spheres are made of PS macromolecules, which were transformed into sphere shapes in the course of phase separation process. According to Fig. 2b, nanosilica and PS macromolecules are both simultaneously present at the top layer of the coating. Since the PS macromolecules are not as hydrophobic as the used nanosilica, the WCA for this sample is lower than that of P1S0.5E15 and P1S1E15, both of which contain much more nanosilica.
Those above-mentioned micro-spheres were also formed in the case of P1S0.5E15 and P1S1E15 samples; however, they were mainly covered with nanosilica particles. The main difference is that the size of spheres is larger for these two samples as compared to P1S0.1E15, which could be attributed to the accelerated phase separation as a result of higher inclusions of nanoparticles (Khakbaz et al. 2015). According to the SEM images, one can state that the incomplete coverage of nanosilica particles on the coating’s top layer is the major reason for the lower WCA value observed in P1S0.1E15. However, the ultra-packed structure of nanosilica in the case of P1S1E15 did not further improve the hydrophobicity as compared to the optimum sample. The reason for this behavior could be ascribed to the use of a very high content of nanosilica, which resulted in a non-uniform coating from the macroscopic viewpoint.
Figure 3 illustrates the surface morphologies for other samples from which several comparisons can be made. First of all, comparing Fig. 3a and Fig. 2c reveals that increasing the non-solvent content from 15 to 25 wt% caused formation of more distinct PS spheres on the top layer of the coating. According to the wettability results, increasing the non-solvent content caused a reduction in the WCA value from 165 to 151°. It was expected that the presence of non-solvent would influence the surface morphology of polymer macromolecules because of the occurrence of the phase separation process. This influence becomes more evident once the non-solvent is absent in the formulation. Figure 3c, d shows the surface morphology of P1Si0.5, which has been fabricated in the absence of non-solvent. It is seen that the surface morphology is severely changed, and many fewer PS spheres were formed, resulting in a rather non-uniform surface roughness, which causes a notable decline in the hydrophobicity of the surface (Seyfi et al. 2015a). Those large and irregularly shaped PS aggregates on the top layer of the coating could act as physical and chemical inhomogeneity, which might lead to higher WCA hysteresis (difference in advancing and receding WCAs) and thus lower hydrophobicity. In fact, this sample exhibited a sticky behavior such that the water droplet was stuck to the filter paper even when the paper turned upside down. This observation confirms the above claim, that is, the irregular PS aggregates can act as inhomogeneity and increase the water stickiness. This result is in agreement with the result of our recent publication in which the uniformity of rough surface features was found to be of great importance (Seyfi et al. 2015a).
The effect of polymer concentration on surface morphology can be clearly observed by comparing Fig. 3e and Fig. 2c. It is seen that a very high amount of polymer in this type of formulation could adversely affect both the surface morphology and hydrophobicity since the WCA was significantly reduced to 101°, which is very close to that of the pure PS coating. According to Fig. 3e, f, it can be assumed that PS macromolecules are abundantly present at the surface’s top layer leading to a very non-uniform surface roughness. The above hypothesis will be later proved via XPS analysis.
Surface morphologies of samples prepared via dip-coating method are presented in Fig. 4. Comparing Fig. 4a and Fig. 3c, one could realize the influence of the coating technique on the surface morphology of P1S0.5. No more traces of PS aggregates can be detected on the surface of P1S0.5_Dip. More interestingly, the surface of P1S0.5E15_Dip is also completely covered by nanosilica particles, which is quite unexpected since non-solvent was present in its formulation. As mentioned earlier, non-solvent highly influences the surface morphology of polymer macromolecules, which are not abundantly present at the surface layer of this sample. Thus, in contrast to the solution casting technique, it can be concluded that the presence of non-solvent has a weaker influence on the surface morphology and thus hydrophobicity of the coatings. Another conclusion is that PS macromolecules exhibited an insignificant tendency to be adsorbed on the surface of filter paper during the dipping process; instead, nanosilica particles were highly adsorbed and resulted in a very uniform and packed morphology (Fig. 4c), which can be considered as the major reason for the observed high WCA value. It should be noted that contrary to the solution casting method, the filter paper is held vertically within the solution; thus, deposition of PS and silica occurred unforcedly. Moreover, the intrinsically porous nature of filter paper facilitates the adsorption of silica nanoparticles rather than PS macromolecules.
Surface composition
X-ray photoelectron spectroscopy (XPS) analysis was utilized to evaluate the changes made in the surface composition of different samples. Figure 5 shows the wide-scan spectra for two samples, which only differed in their polymer content (P1S0.5E15 and P5S0.5E15). It was previously observed that the surface morphology of the optimum sample was significantly changed upon using a higher amount of PS, which caused a dramatic reduction in the WCA value. Apart from that, the changes in surface composition could also be regarded as another reason. Figure 5a shows the XPS spectrum for the optimum sample (P1S0.5E15), indicating the presence of O, C and Si atoms on its top layer. The most prominent atom on the surface of the optimum sample is oxygen, indicating the strong aggregation of nanosilica (SiO2) particles on the coating’s surface. According to Fig. 5a, PS macromolecules are also present at the surface layer. In contrast, once the polymer content is increased, the majority of the surface layer is composed of carbon atoms, implying the more profound presence of PS macromolecules on the top layer of the coating.
The quantified XPS results are also reported in Table 2. The surface composition of unmodified filter paper is also reported. However, in the case of unmodified paper, the oxygen atoms belong to the cellulose polymer instead of silica molecules since the used filter paper is made of cellulose. It is seen that the carbon atomic content of the optimum sample is doubled upon the increment of PS content from 1 to 5 wt%. On the other hand, the Si and O atomic contents are both decreased, which suggests the aggregation of PS macromolecules on the top layer of the coating in the case of P5S0.5E15. Therefore, the claim made earlier based on SEM observations is now corroborated. In fact, such a strong presence of PS macromolecules on the surface layer could be regarded as responsible for the obtained WCA value (101°), which is very close to that of pure PS (98°).
According to Table 2, one could observe that the presence of non-solvent plays an important role in the surface composition of the coatings since P1S0.5 exhibits a notably higher content of carbon atoms with respect to the optimum sample (P1S0.5E15). The results reveal that the nanosilica particles were less aggregated on the surface of P1S0.5, which can be considered as the main reason for the obtained low WCA value (112°). Therefore, apart from the induced non-uniformity in the surface morphology of P1S0.5, the surface energy is also highly influential on the final wetting behavior. In other words, the weaker presence of hydrophobic nanosilica particles on the surface resulted in a higher surface energy as compared with P1S0.5E15, which caused the WCA to be severely decreased.
Oil/water separation
At first, it is worth determining the adsorption percentages of PS macromolecules and silica nanoparticles. The weights of both parent and modified filter papers were measured, and the adsorption percentage was obtained based on the following equation:
The results are reported in Table 3. It is seen that the optimum formulation was interestingly found to exhibit a significantly higher adsorption value once the solution casting technique had been employed. In other words, the dip-coating technique caused a great reduction in the adsorption value. This finding is in total agreement with the suggested hypothesis based on the SEM images shown in Fig. 4, that is, many fewer PS macromolecules were adsorbed to the paper’s surface via the dip-coating technique; instead, nanoparticles showed a good level of adsorption. Based on the obtained adsorption values for P1S0.5E15 (11.5 %) and P1S0.5 (6.5 %), it can be concluded that non-solvent plays a vital role in the adsorption values by improving the phase separation process, which leads to deposition of more amounts of polymer chains on the surface of the paper.
The thickness of the modified filter papers was also measured via a digital micrometer upon performing the measurements before and after the coating process. The uniformity of the coating was ensured by measuring the thicknesses at different positions. It was found that the coating thickness for P1S0.5E15 was 14.3 µm, whereas it was 8.9 µm for P1S0.5E15_Dip. These results are in conformity with the adsorption values reported in Table 3. It could be inferred that the dipping technique resulted in a thinner coating that was mainly composed of silica nanoparticles. On the other hand, the solution casting technique resulted in a thicker coating wherein PS macromolecules were abundantly adsorbed on the filter paper’s surface.
Figure 6a shows that the filter paper, modified by the optimum formulation, exhibits a superoleophilic property; thus, the crude oil droplet is completely absorbed by the paper. On the other hand, Fig. 6b demonstrates the ability of modified filter paper to repel water. Combining these two properties, one could design efficient and simple systems for the separation of oil from water. An example is given in Fig. 6d where the whole filter paper was surface modified by the presented method in this study and used as a separator of crude oil from water. The efficiency of separation was also determined using the following formula:
where v 0 and v are the volumes of water before and after the separation process, respectively. Having v 0 as 5 ml and v as 4.95 ml, the separation efficiency would be as high as 99 %.
The thermal stability of the imparted superhydrophobic property was also investigated by placing the modified filter paper in an oven (200 °C) for 2 h. Figure 6c shows that the superhydrophobicity is still retained (WCA = 158 ± 3, SA = 8°) after being severely heated, indicating the excellent resistance of the induced structure at the coating’s top layer of the optimum sample. Figure 7a–c also shows the ability of the modified filter paper in the absorption of motor oil from an oil/water mixture. In order to prove the efficiency of the separation and the fact that the modified paper only absorbs the oily phase, sequential images of soaking the modified paper into the water are illustrated in Fig. 7d–f. It is seen that the modified filter paper remains completely dry after immersion into the water bath.
Conclusions
In this work, the surface of filter paper was modified by polystyrene and nanosilica via an improved phase separation method that involves the use of both non-solvent and nanoparticles simultaneously. The role of non-solvent was essential in achieving the superhydrophobic property considering that the nanocomposites made in the absence of non-solvent did not exhibit superhydrophobic behavior. It was found that an extremely high content of polymer had an adverse effect on the hydrophobicity of the papers. It was also demonstrated that the dip-coating technique is not as efficient as the solution casting in preparation of stable superhydrophobic coatings. According to the SEM results, it was hypothesized that PS macromolecules were less likely to be adsorbed onto the surface of filter papers; however, nanosilica particles exhibited a very good adsorption. XPS analysis was used to prove the above-mentioned hypothesis and showed that the extent of polymer adsorption was reduced on the filter papers. All in all, it could be deduced that both the surface roughness and surface chemistry should be adjusted in a certain range to achieve superhydrophobicity. It was shown that the hydrophobically modified silica changed the surface chemistry while the addition of non-solvent during the fabrication process enhanced the surface roughness. Therefore, the simultaneous incorporation of nanoparticle and non-solvent acted synergistically to impart superhydrophobic behavior onto the intrinsically hydrophilic filter papers. Finally, the experimental results showed that this type of formulation could have promising potentials in oil/water separation applications.
References
Arbatan T, Zhang L, Fang X-Y, Shen W (2012) Cellulose nanofibers as binder for fabrication of superhydrophobic paper. Chem Eng J 210:74–79
Böhm A, Gattermayer M, Trieb C et al (2013) Photo-attaching functional polymers to cellulose fibers for the design of chemically modified paper. Cellulose 20:467–483
Chen D, Chen F, Zhang H et al (2016) Preparation and characterization of novel hydrophobic cellulose fabrics with polyvinylsilsesquioxane functional coatings. Cellulose 23:941–953
Du C, Wang J, Chen Z, Chen D (2014) Durable superhydrophobic and superoleophilic filter paper for oil–water separation prepared by a colloidal deposition method. Appl Surf Sci 313:304–310
Feng L, Zhang Z, Mai Z et al (2004) A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water. Angew Chem Int Ed 43:2012–2014
Gao Z, Zhai X, Liu F et al (2015) Fabrication of TiO 2/EP super-hydrophobic thin film on filter paper surface. Carbohydr Polym 128:24–31
Hejazi I, Sadeghi GMM, Jafari SH et al (2015a) Transforming an intrinsically hydrophilic polymer to a robust self-cleaning superhydrophobic coating via carbon nanotube surface embedding. Mater Des 86:338–346
Hejazi I, Seyfi J, Hejazi E et al (2015b) Investigating the role of surface micro/nano structure in cell adhesion behavior of superhydrophobic polypropylene/nanosilica surfaces. Colloids Surf B Biointerfaces 127:233–240
Hejazi I, Sadeghi GMM, Seyfi J et al (2016) Self-cleaning behavior in polyurethane/silica coatings via formation of a hierarchical packed morphology of nanoparticles. Appl Surf Sci 368:216–223
Hsu S-H, Woan K, Sigmund W (2011) Biologically inspired hairy structures for superhydrophobicity. Mater Sci Eng R Rep 72:189–201
Khakbaz M, Hejazi I, Seyfi J et al (2015) A novel method to control hydrolytic degradation of nanocomposite biocompatible materials via imparting superhydrophobicity. Appl Surf Sci 357:880–886
Li H, Zhao X, Wu P et al (2016) Facile preparation of superhydrophobic and superoleophilic porous polymer membranes for oil/water separation from a polyarylester polydimethylsiloxane block copolymer. J Mater Sci 51:3211–3218
Meng Y, Young TM, Liu P et al (2015) Ultralight carbon aerogel from nanocellulose as a highly selective oil absorption material. Cellulose 22:435–447
Ogihara H, Xie J, Saji T (2013) Factors determining wettability of superhydrophobic paper prepared by spraying nanoparticle suspensions. Colloids Surf Physicochem Eng Asp 434:35–41
Seyfi J, Hejazi I, Jafari SH et al (2015a) On the combined use of nanoparticles and a proper solvent/non-solvent system in preparation of superhydrophobic polymer coatings. Polymer 56:358–367
Seyfi J, Jafari SH, Khonakdar HA et al (2015b) Fabrication of robust and thermally stable superhydrophobic nanocomposite coatings based on thermoplastic polyurethane and silica nanoparticles. Appl Surf Sci 347:224–230
Seyfi J, Hejazi I, Jafari SH, Khonakdar HA, Simon F (2016) Enhanced hydrophobicity of polyurethane via non-solvent induced surface aggregation of silica nanoparticles. J Colloid Interface Sci 478:117–126
Shen J, Song Z, Qian X, Ni Y (2010) A review on use of fillers in cellulosic paper for functional applications. Ind Eng Chem Res 50:661–666
Siegel AC, Phillips ST, Wiley BJ, Whitesides GM (2009) Thin, lightweight, foldable thermochromic displays on paper. Lab Chip 9:2775–2781
Tang Z, Hess DW, Breedveld V (2015) Fabrication of oleophobic paper with tunable hydrophilicity by treatment with non-fluorinated chemicals. J Mater Chem A 3:14651–14660
Teisala H, Tuominen M, Haapanen J et al (2014) Switchable water absorption of paper via liquid flame spray nanoparticle coating. Cellulose 21:2033–2043
Thorvaldsson A, Edvinsson P, Glantz A et al (2012) Superhydrophobic behaviour of plasma modified electrospun cellulose nanofiber-coated microfibers. Cellulose 19:1743–1748
Wang S, Li M, Lu Q (2010) Filter paper with selective absorption and separation of liquids that differ in surface tension. ACS Appl Mater Interfaces 2:677–683
Xue Z, Cao Y, Liu N et al (2014) Special wettable materials for oil/water separation. J Mater Chem A 2:2445–2460
Yuan J, Liu X, Akbulut O et al (2008) Superwetting nanowire membranes for selective absorption. Nat Nanotechnol 3:332–336
Zangi S, Hejazi I, Seyfi J et al (2016) Tuning cell adhesion on polymeric and nanocomposite surfaces: role of topography versus superhydrophobicity. Mater Sci Eng, C 63:609–615
Zhang M, Wang C, Wang S et al (2012) Fabrication of coral-like superhydrophobic coating on filter paper for water–oil separation. Appl Surf Sci 261:764–769
Zhang J, Ji K, Chen J et al (2015) A three-dimensional porous metal foam with selective-wettability for oil–water separation. J Mater Sci 50:5371–5377
Acknowledgments
Partial financial support from the Iranian Nanotechnology Initiative is gratefully appreciated.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Piltan, S., Seyfi, J., Hejazi, I. et al. Superhydrophobic filter paper via an improved phase separation process for oil/water separation: study on surface morphology, composition and wettability. Cellulose 23, 3913–3924 (2016). https://doi.org/10.1007/s10570-016-1059-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-1059-y