Abstract
In this study, the effect of alkaline pretreatment on preparation of regenerated lignocellulose fibers from bamboo stem was studied in detail. Prior to being dissolved in [Emim]OAc, bamboo stems were ground and treated with different concentrations of sodium hydroxide solutions. The obtained spinning dopes were then extruded into water coagulation bath and regenerated to composite fibers. The properties of the raw materials, spinning dopes, and regenerated fibers were investigated. Results showed that alkaline pretreatment could break the rigid structure of lignocelluloses by removing part of hemicelluloses and lignin as well as changing the polymorphous lattice and C r I of cellulose. The increased specific surface area of raw materials enhanced the accessibility of solvent, promoted the swelling process and shortened the dissolution time. The viscosity of the spinning dopes and the strength of the regenerated fiber reached the maximum value when the bamboo powder was treated with 20 wt% sodium hydroxide at 60 °C for 4 h. In addition, the fibers prepared from the alkali-treated raw materials showed round cross-section and wrinkled surface. Therefore, the properties of the regenerated lignocellulose fibers could be improved by employing an appropriate alkali pretreatment of raw materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the growing emphasis on sustainable development and environmental conservation, the utilization of abundant and renewable biomass resources has attracted great attentions (Huber et al. 2006; Ragauskas et al. 2006). The primary component of plant biomass is lignocellulose, which is composed of three major biopolymers: cellulose, hemicelluloses, and lignin (Loerbroks et al. 2013; Maki-Arvela et al. 2010; Peng et al. 2012). The ordered cellulose chains formed tightly packed crystalline regions of cellulose microfibrills, which are embedded within a matrix of hemicelluloses and lignin (Boudet et al. 2003). In addition, lignin and carbohydrates are linked by covalent bonds (lignin–carbohydrate complexes, LCCs) (Du et al. 2013). This complex and inaccessible structure causes the difficult fractionation and dissolution of lignocellulosic biomass (Zakzeski et al. 2010).
Delignification processes are usually used to extract cellulose from lignocellulosic biomass for paper making industry. The pulping techniques are primarily focused on maximizing the cellulose yield, while most of the hemicelluloses and lignin are wasted. In addition, the use of chemicals usually causes environmental pollution (Sun et al. 2011b). It has been demonstrated that the dissolution of lignocellulosic biomass without prior isolation of individual components could offer a variety of new possibilities for converting biomass to valuable biofuels, chemicals, and biomaterials (Kilpelainen et al. 2007). The major difficulty in dissolving lignocellulosic biomass is to cleave the primary bonds between the biopolymer components, including the covalent bonds between lignin and carbohydrates as well as the inter- and intramolecular hydrogen bonding in cellulose chains (Brandt et al. 2013). Thus, it is hard to directly dissolve lignocellulosic biomass in many conventional solvents. Ionic liquids (ILs), especially 1-ethyl-3-methyl-imidazolium acetate ([Emim]OAc), have been considered as effective solvents for lignocellulosic biomass (Zavrel et al. 2009). Rice straw, switchgrass, corn stalks, pine, spruce, and other biomass materials have been dissolved in ILs (Brandt et al. 2013; Chen et al. 2014; Fort et al. 2007; Kilpelainen et al. 2007; Kyllonen et al. 2013; Lee et al. 2009; Wang et al. 2014). The main purpose of this dissolution is to use them as a pretreatment to enhance the enzymatic hydrolysis of cellulose by selective extraction of lignin, decreasing the crystallinity of cellulose, and increasing the accessibility of the polysaccharide chains to cellulases (Isikgor and Becer 2015).
The dissolution of lignocellulosic biomass in ILs not only enriches the pretreatment techniques, also promotes the development of biomaterials, as valuable materials can be directly prepared from lignocellulosic biomass solutions by regenerating process (Muhammad et al. 2012). Cellulose–hemicelluloses–lignin composite fibers and films have been prepared from wood and bagasse through the dissolution and regeneration processes (Chen et al. 2014; Simmons et al. 2010; Sun et al. 2011a). The preparation of these composite materials without any pulping or pretreatment step provides a simple and efficient route to fully use lignocellulosic biomass. However, the strength of these composite materials is much lower than that of the materials prepared only from cellulose. In previous reports (Sun et al. 2011a; Kang et al. 2013), it was reported that alkaline pretreatment of pine and hemp before dissolution could improve the strength and spinnability of the obtained biomass composite materials. To comprehensively understand this improvement, the effect of alkaline pretreatment on preparation process needs to be studied in detail.
In this study, bamboo, which is a large woody grass and widely distributed in the Asian countries, was chosen as raw material. The bamboo stems were ground and pretreated with different concentrations of sodium hydroxide solution. The treated and untreated bamboo powders were then dissolved in [Emim]OAc at a high temperature and extruded into coagulation bath to prepare regenerated lignocellulose fibers, respectively. The properties of the materials, dopes and fibers were determined. The purpose of this study was to give a comprehensive understanding of the effect of alkali pretreatment on raw material, dissolving process, and fibers’ properties during the preparation of lignocellulosic composite fibers.
Experimental
Materials and reagents
Bamboo stems (Neosinocalamus affinis), which were obtained from Sichuan province, China, were cut into small pieces (1–3 cm) and ground in a mill to pass through an 80-mesh sieve. The particles were then dried in an oven at 60 °C for 24 h and kept in a desiccator for further use. The ionic liquid, [Emim]OAc, was acquired from Lanzhou Institute of Chemical Physics, Lanzhou, China. Other chemicals were of analytical or reagent grade and used as purchased without further purification.
Alkaline pretreatment
The bamboo powders, named as B0, were extracted by 0, 5, 10, 15, 20, 25, 30 and 35 wt% NaOH aqueous solutions at 60 °C for 4 h with a solid to liquor ratio of 1:20 (g/ml), respectively. After filtration, the solid residues were washed to neutral with water, oven-dried and labeled as B5, B10, B15, B20, B25, B30 and B35, respectively.
Preparation of the lignocellulose fibers
9.5 g of [Emim]OAc were loaded into a 25 mL round-bottom flask and heated to 175 °C under nitrogen atmosphere with an oil bath. Then 0.5 g untreated or pretreated bamboo powders were carefully added into the solvent under vigorous stirring to obtain a spinning dope. After being degassed under vacuum, the dope was transferred to a syringe and extruded through an orifice of 0.21 mm diameter into a water coagulation bath at 25 °C by wet spinning method. The extrusion velocity was 1.2 m/min. The regenerated fibers were washed several times with water and soaked in water overnight to remove the residual solvent. The wet fibers were then collected by a spool to prevent shrinkage and air dried without stretching. The obtained fibers were labeled as F0, F5, F10, F15, F20, F25, F30 and F35, respectively.
Characterization
The chemical compositions (%, w/w) of all the bamboo powders were determined by National Renewable Energy Laboratory’s (NREL) standard analytical procedure. The carbohydrates of the materials were analyzed by a high-performance anion-exchange chromatography (HPAEC) (Dionex, ISC 3000, USA) system on a CarboPac PA 20 (Dionex, USA) analytical column. The XRD patterns of the raw materials were measured by X-ray diffraction on an XRD-6000 instrument (Shimadzu, Japan) with a scattering angle (2θ) from 5° to 40° at a scanning speed of 0.2°/min. The crystallinity index (C r I) values were calculated according to the method described in a previous paper (Chen et al. 2015b).
The morphology of the bamboo powders and regenerated lignocellulose fibers was observed by field emission scanning electron microscopy (FESEM) (SU8010, HITACHI, Japan) at acceleration voltages of 5 kV after being sputtered with gold–palladium in a sputter coater (E-1010, HITACHI, Japan). To obtain the cross-section microscopy, the fibers were fractured in liquid nitrogen before being sputtered with gold–palladium. The three-dimensional (3D) surface topography of the fibers were measured using a Nanoscope IIIa Multimode scanning probe microscope (Digital Instruments Inc., USA). The atomic force microscopy (AFM) images were scanned using tapping mode regime with silicon cantilevers at room temperature in air. The images were only flattened without any other processing.
After the addition of the bamboo powders in the solvent, the dissolving process was observed by a Leica DM2500 fluorescence microscope (Leica Microsystems, Germany) at the set time. The viscosities of the spinning dopes were measured on a Brookfield digital viscometer DV-II + PRO (Brookfield Instruments, America) using S34 rotor with a speed of 5.0 rpm at 80 °C. The tensile strength (σ b ) and elongation at break (ε b ) of the regenerated fibers were measured on a universal tensile tester (UTM6503, Shenzhen Suns Technology stock CO. LTD. China) according to ASTM D2256-80. The fiber samples were preconditioned at 20 °C and 65 % relative humidity (RH) for 24 h. A gauge length of 20 mm and a speed of 2.0 mm/min were used for the test.
Results and discussion
Effect of alkaline pretreatment on the properties of raw materials
Alkaline treatment has been considered as a traditional and efficient way to disrupt the lignin–carbohydrate matrix, and has been extensively used to remove hemicelluloses, lignin, and/or cellulose from lignocellulosic biomass (Eronen et al. 2009; Kotarska et al. 2015; Park and Kim 2012; Zheng et al. 2014). Alkaline pre-treatment at 60 °C for 4 h has been considered as an effective procedure to break the rigid structure of lignocelluloses and changes the polymorphic lattice of cellulose (Viell 2014). In this study, alkaline pretreatment was employed to pretreat bamboo powders to enhance their dissolution in ionic liquid. The sugar components of the raw materials are listed in Table 1. The composition of untreated bamboo powders was 43.04 % cellulose (expressed as glucan), 22.13 % hemicelluloses (composed of xylan, arabinan, and galactan), and 27.14 % lignin (25.12 % Klason lignin and 2.02 % acid-soluble lignin). After alkaline pretreatment, the contents of hemicelluloses (10.28–16.89 %) and lignin (17.57–22.98 %) of the bamboo powders were obviously decreased, meanwhile, the content of cellulose was gradually increased (51.18–62.47 %). It was probably due to the fact that the hydrolysable linkages, such as α- and β-aryl ethers in lignin and glycosidic bonds in hemicelluloses, were partly cleaved by alkaline treatment, resulting in partial removal of hemicelluloses and lignin (McIntosh and Vancov 2010; Wen et al. 2011).
The chemical structure of the treated and untreated bamboo powders was investigated by XRD (Fig. 1). The crystal structure of the bamboo powders changed from cellulose I to cellulose II after being treated by alkali solution with a concentration of 15 wt%. The slightly increased CrI value of the bamboo powder treated by 5 wt% NaOH could be attributed to the removal of non-crystalline lignin and hemicelluloses. The CrI value was then decreased when further increased the alkali concentration, suggesting that part of the crystalline region of cellulose was destroyed.
The morphology of the raw materials before and after alkali pretreatment was investigated by SEM (Fig. 2). Big bundles with intact and rigid structure were observed in untreated bamboo powders (B0). After being treated by alkali, the big bundles were gradually cleaved and dissociated, and fibrils buried in these bundles were gradually exposed with the removal of adhesive substances between them (B0 and B10). When alkali concentration was higher than 20 wt%, the fibrils were completely isolated (B15). From the enlarged images (B0a–B15a), untreated bundles had a relatively smooth surface, while the isolated fibrils showed a surface with lots of fine wrinkles. The formation of wrinkles was investigated by observing the cleavage of the bundles at a high magnification (Fig. 3). After the superficial substances, probably consisted of hemicelluloses and lignin according to the composition analysis, were gradually removed, fibrils buried in bundles were exposed. The isolation of fibrils could be considered as a kind of stripping and tearing process, as microfibrils were clearly observed between them (A and B). From the amplified images (C and D), these microfibrils had a diameter less than 50 nm and were consisted of aggregations of the nanofibrils that wrapped on fibrils’ surface. Under external shear force, the microfibrils were eventually broken, leading to the formation of wrinkles on the surface of fibrils. Thus, the specific surface area of the raw materials was significantly increased during the alkaline pretreatment.
Dissolution of the untreated and alkali-treated bamboo powders
Among ILs, [Emim]OAc has been considered as one of the most efficient solvents for lignocellulosic biomass dissolution. This may be related to the basicity of the acetate anion which could disrupt the intricate network of non-covalent interactions among hemicelluloses, cellulose, and lignin, as well as the inter- and intra-molecular hydrogen bonding in biopolymers (FitzPatrick et al. 2012). However, i (ca. 15–16 h) to achieve complete dissolution of the untreated wood powders in [Emim]OAc at 110 °C (Sun et al. 2009). It has been reported that rapid dissolution could be reached by using a dissolving temperature above the glass transition of lignin (Li et al. 2011). In this study, the untreated bamboo powder was completely dissolved in 20 min under a relatively high dissolution temperature (175 °C) (Table 2). This is even shorter than the time (1.5 h) needed for delignified bamboo cellulose (M w = 1353,500) dissolved in the same solvent at 110 °C (Chen et al. 2015a). The dissolution time was obviously shortened for the alkali-treated bamboo powders. This was probably due to the increased accessibility of the raw materials to solvent.
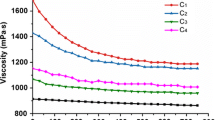
During the dissolution process, the viscosity of the powders–solvent mixture was increased at first, and then gradually decreased. Large amount of insoluble fibers could be observed with naked eyes when the viscosity was highest. The change of viscosity was closely related to the status of bamboo powders in ionic liquids. From Fig. 4, it could be clearly observed that the bamboo powders were fully swelled before been dissolved. Thus, the swelling of the raw material caused the increase of viscosity. When most of the raw material was dissolved, a liquid suspension was finally formed. This suspension was not homogeneous but it did not affect the spinning process, as Michels and Kosan (2006) demonstrated the existence of only partial dissolved cellulose particles even if highly purified dissolving pulps are used for dope preparation in [Emim]OAc. The swelling and dissolving processes were more efficient for the alkali-treated bamboo powders. This was probably due to the increased specific surface area of the raw materials. It is noteworthy that some substances existed in the untreated raw materials were difficult to be dissolved. From the optical microscopic images (Fig. 5, left), these substances had compact structure. As lignin is fluorescent due to its phenolic structural units (De Micco and Aronne 2007), the strong fluorescence in the fluorescence microscopic images (Fig. 5, right) indicated that they had a high content of lignin. Therefore, it could be deduced that alkali treatment promoted the swelling process and shorten the dissolution time by removing part of insoluble substances and increasing the specific surface area of the raw material.
Alkali pretreatment also influenced the viscosity of the dopes obtained (Table 2). The highest viscosity was reached when bamboo powders were treated by 20 wt% NaOH at 60 °C for 4 h. This increment could be associated with the dissolution time of the raw materials in solvent. It has been found that the pre-treatment of cellulose at elevated caustic soda concentrations causes noticeable conversion of cellulose I to cellulose II, and cellulose II could be much faster dissolved in anhydrous [Emim]OAc (Schleicher et al. 1985). As known, degradation of biomass components in ionic liquids is inevitable, and the degree of this degradation is related to the dissolution time and temperature (FitzPatrick et al. 2012; Lu et al. 2013; Michud et al. 2015; Pan et al. 2014; Zhou et al. 2012). The high dissolution temperature used in this study undoubtedly promoted the degradation of the raw material. Therefore, short dissolution time could reduce the degree of degradation, resulting in a high viscosity of the dope. It should be pointed out that the degradation of raw materials not only occurred in dissolving process, also took place in the process of alkali pretreatment, and serious degradation was more likely happened at a high alkali concentration. Thus, the viscosity of the dopes was decreased when using B25, B30, and B35 as the raw materials.
Structural and mechanical properties of the regenerated lignocellulose fibers
Due to the existence of lignin, degradation of (hemi-)cellulose and concomitant chromophore formation, the color of the dope changed from light yellow to dark brown (Fig. 6a, b). After being regenerated, the obtained fibers showed a brown color (Fig. 6c). From the optical and fluorescence microscopic images (Fig. 6d, e), lignin was uniformly distributed in the regenerated lignocellulose fibers.
The cross-section morphology of the regenerated fibers is shown in Fig. 7. It can be clearly observed that the fiber prepared from the untreated bamboo powders (F0) showed an irregular cross section with a diameter of about 80 μm, while the fibers prepared from the pretreated bamboo powders (F35) showed a circular cross section with a diameter of about 50 μm. The diameter of the fibers was decreased with the increase of cellulose contents, because high cellulose content could promote the fiber radial shrinkage by forming a compact hydrogen bond network structure in the drying process. From the magnified images (F0a–F35a), the internal structure of the fiber was relatively homogeneous and dense without any defects was revealed. As shown in Fig. 8, the surface of the fiber F0 was uneven and distributed with lots of long narrow cracks, while other fibers F5–35 showed a roughen and striate surface. Cracks were also observed on the surface of fibers F5–35, except F15. The 3D surface topography of the fibers over a selected area of 1 μm × 1 μm are shown in Fig. 9. The surface of F0 was smoother than that of the fibers F15 and F35. As all the spinning dopes were initially extruded from the same round spinneret, the variety of morphology might be ascribed to the different diffusion rate of components during coagulating process. As known, cellulose and hemicelluloses are hydrophilic due to their hydroxyl groups while lignin is hydrophobic due to its phenyl groups. In the coagulation bath, the regenerated rate of these components may be different due to the opposite polarity. However, further research is needed to confirm this deduce.
The tensile strength (σ b ) and elongation at break (ε b ) of the regenerated lignocellulose fibers prepared from the untreated bamboo powders were 0.46 cN/dtex and 5.53 %, respectively (Table 2). The mechanical properties of the fibers prepared from bamboo powders treated with 20 wt% NaOH were increased to 1.05 cN/dtex (σ b ) and 9.45 % (ε b ), respectively, indicating that the alkaline pretreatment was an effective method to improve the strength of the regenerated fibers. This could be attributed to the increased average molecular weight of the raw materials due to the removal of hemicelluloses and lignin. On the other hand, the increase of cellulose content in the spinning dope could promote the orientation of the regenerated fiber, leading to the improvement of the fibers’ mechanical strength. However, when alkali concentration was higher than 20 wt%, the strength of the obtained fiber was decreased. This should be ascribed to the decreased average molecular weight caused by the degradation of components especially cellulose in the raw materials. The reducing end aldehyde groups of cellulose molecular chains were removed one by one under the strong alkali condition, leading to the degradation of cellulose chains. In addition, the yield of the raw materials (Table 1) and the water consumption were also needed to be considered during the pretreatment. Therefore, the appropriate alkali concentration for treatment of bamboo powders was 20 wt% in this study. This dosage of sodium hydroxide can be further reduced by raising the temperature and extending the processing time, which will be researched in the subsequent studies. In addition, sodium hydroxide in the waste stream and ionic liquids in the coagulation bath could be recycled after filtration and concentration. However, the use of [Emim]OAc at 175 °C could cause a strong solvent decomposition and undesired side reactions of solvent and materials (Dorn 2009). The remained components and the soluble oligomers resulted from the degradation of the compounds in the IL–water spin bath mixture increased the difficulty of solvent recovery. Therefore, the effective recycling of ionic liquids is also an important problem need to be considered when using holo-cellulose or lignocellulose as feedstock to produce regenerated composite fibers.
Conclusions
In this study, the effect of alkali pretreatment on the raw material, dissolving process, and fibers’ properties during the preparation of regenerated lignocellulose fibers from bamboo stem was investigated in detail. Results showed that alkali pretreatment increased the cellulose content and broke the compact structure of the raw materials. The improvement of solvent accessibility promoted the swelling process and shortened the dissolution time. The viscosity of spinning dopes was first increased and then decreased with the increment of alkali concentration. The fibers prepared from the alkali-treated bamboo powders showed round cross section and wrinkled surface. The mechanical properties of these fibers were increased by 128.3 % (σ b ) and 70.9 % (ε b ), respectively, compared to that of the fibers prepared from the untreated materials. Therefore, alkaline pretreatment is an effective way to improve the properties of regenerated lignocellosic fibers and the appropriate alkali concentration for pretreatment of bamboo powder was 20 wt%.
References
Boudet AM, Kajita S, Grima-Pettenati J, Goffner D (2003) Lignins and lignocellulosics: a better control of synthesis for new and improved uses. Trends Plant Sci 8:576–581. doi:10.1016/j.tplants.2003.10.001
Brandt A, Gräsvik J, Hallett JP, Welton T (2013) Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem 15:550–583. doi:10.1039/c2gc36364j
Chen M, Zhang X, Liu C, Sun R, Lu F (2014) Approach to renewable lignocellulosic biomass film directly from bagasse. ACS Sustain Chem Eng 2:1164–1168. doi:10.1021/sc400555v
Chen JH, Guan Y, Wang K, Zhang XM, Xu F, Sun RC (2015a) Combined effects of raw materials and solvent systems on the preparation and properties of regenerated cellulose fibers. Carbohydr Polym 128:147–153
Chen JH, Guan Y, Wang K, Xu F, Sun RC (2015b) Regulating effect of hemicelluloses on the preparation and properties of composite Lyocell fibers. Cellulose 22:1505–1516
De Micco V, Aronne G (2007) Combined histochemistry and autofluorescence for identifying lignin distribution in cell walls. Biotech Histochem 82:209–216
Dorn S (2009) Ionische Flüssigkeiten: Neue Lösemittel und Reaktionsmedien in der Cellulosechemie, PhD Thesis FSU Jena
Du XY, Gellerstedt G, Li JB (2013) Universal fractionation of lignin-carbohydrate complexes (LCCs) from lignocellulosic biomass: an example using spruce wood. Plant J 74:328–338
Eronen P, Österberg M, Jääskeläinen A-S (2009) Effect of alkaline treatment on cellulose supramolecular structure studied with combined confocal Raman spectroscopy and atomic force microscopy. Cellulose 16:167–178. doi:10.1007/s10570-008-9259-8
FitzPatrick M, Champagne P, Cunningham MF (2012) Quantitative determination of cellulose dissolved in 1-ethyl-3-methylimidazolium acetate using partial least squares regression on FTIR spectra. Carbohydr Polym 87:1124–1130
Fort DA, Remsing RC, Swatloski RP, Moyna P, Moyna G, Rogers RD (2007) Can ionic liquids dissolve wood? Processing and analysis of lignocellulosic materials with 1-n-butyl-3-methylimidazolium chloride. Green Chem 9:63. doi:10.1039/b607614a
Huber GW, Iborra S, Corma A (2006) Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem Rev 106:4044–4098
Isikgor FH, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem 6:4497–4559
Kang Y, Ahn Y, Lee SH, Hong JH, Ku MK, Kim H (2013) Lignocellulosic nanofiber prepared by alkali treatment and electrospinning using ionic liquid. Fiber Polym 14:530–536
Kilpelainen I, Xie H, King A, Granstrom M, Heikkinen S, Argyropoulos DS (2007) Dissolution of wood in ionic liquids. J Agr Food Chem 55:9142–9148. doi:10.1021/jf071692e
Kotarska K, Swierczynska A, Dziemianowicz W (2015) Study on the decomposition of lignocellulosic biomass and subjecting it to alcoholic fermentation Study on the decomposition of lignocellulosic biomass. Renew Energ 75:389–394
Kyllonen L, Parviainen A, Deb S, Lawoko M, Gorlov M, Kilpelainen I, King AWT (2013) On the solubility of wood in non-derivatising ionic liquids. Green Chem 15:2374–2378. doi:10.1039/c3gc41273c
Lee SH, Doherty TV, Linhardt RJ, Dordick JS (2009) Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol Bioeng 102:1368–1376
Li WY, Sun N, Stoner B, Jiang XY, Lu XM, Rogers RD (2011) Rapid dissolution of lignocellulosic biomass in ionic liquids using temperatures above the glass transition of lignin. Green Chem 13:2038–2047
Loerbroks C, Rinaldi R, Thiel W (2013) The electronic nature of the 1,4-glycosidic bond and its chemical environment: DFT insights into cellulose chemistry. Chem-Eur J 19:16282–16294. doi:10.1002/chem.201301366
Lu F, Song J, Cheng BW, Ji XJ, Wang LJ (2013) Viscoelasticity and rheology in the regimes from dilute to concentrated in cellulose 1-ethyl-3-methylimidazolium acetate solutions. Cellulose 20:1343–1352
Maki-Arvela P, Anugwom I, Virtanen P, Sjoholm R, Mikkola JP (2010) Dissolution of lignocellulosic materials and its constituents using ionic liquids-a review. Ind Crop Prod 32:175–201. doi:10.1016/j.indcrop.2010.04.005
McIntosh S, Vancov T (2010) Enhanced enzyme saccharification of Sorghum bicolor straw using dilute alkali pretreatment. Bioresour Technol 101:6718–6727
Michels C, Kosan B (2006) Beitrag zur Struktur von Lyocellfasern, ersponnen aus Aminoxidhydraten bzw. ionischen Flüssigkeiten. Lenzinger Ber 86:144–153
Michud A, Hummel M, Haward S, Sixta H (2015) Monitoring of cellulose depolymerization in 1-ethyl-3-methylimidazolium acetate by shear and elongational rheology. Carbohydr Polym 117:355–363
Muhammad N, Man Z, Khalil MAB (2012) Ionic liquid—a future solvent for the enhanced uses of wood biomass. Eur J Wood Wood Prod 70:125–133
Pan JY, Fu J, Deng SG, Lu XY (2014) Microwave-assisted degradation of lignin model compounds in imidazolium-based ionic liquids. Energ Fuel 28:1380–1386
Park YC, Kim JS (2012) Comparison of various alkaline pretreatment methods of lignocellulosic biomass. Energy 47:31–35
Peng F, Peng P, Xu F, Sun RC (2012) Fractional purification and bioconversion of hemicelluloses. Biotechnol Adv 30:879–903
Ragauskas AJ et al (2006) The path forward for biofuels and biomaterials. Science 311:484–489
Schleicher H, Philipp B, Kunze J, Fink HP (1985) Neue Ergebnisse zur Bildung und Reaktionsweise von Alkalicellulose. Lenzinger Ber 59:45–51
Simmons TJ et al (2010) Preparation of synthetic wood composites using ionic liquids. Wood Sci Technol 45:719–733. doi:10.1007/s00226-010-0395-6
Sun N, Rahman M, Qin Y, Maxim ML, Rodríguez H, Rogers RD (2009) Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem 11:646. doi:10.1039/b822702k
Sun N, Li W, Stoner B, Jiang X, Lu X, Rogers RD (2011a) Composite fibers spun directly from solutions of raw lignocellulosic biomass dissolved in ionic liquids. Green Chem 13:1158–1161. doi:10.1039/c1gc15033b
Sun N, Rodriguez H, Rahman M, Rogers RD (2011b) Where are ionic liquid strategies most suited in the pursuit of chemicals and energy from lignocellulosic biomass? Chem Commun 47:1405–1421. doi:10.1039/c0cc03990j
Viell J (2014) A pre-treatment process of wood based on ionic liquids. Rwth Aachen 23:15–19
Wang H et al (2014) Physical insight into switchgrass dissolution in ionic liquid 1-ethyl-3-methylimidazolium acetate. ACS Sustain Chem Eng 2:1264–1269
Wen JL, Xiao LP, Sun YC, Sun SN, Xu F, Sun RC, Zhang XL (2011) Comparative study of alkali-soluble hemicelluloses isolated from bamboo (Bambusa rigida). Carbohydr Res 346:111–120. doi:10.1016/j.carres.2010.10.006
Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM (2010) The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110:3552–3599
Zavrel M, Bross D, Funke M, Buchs J, Spiess AC (2009) High-throughput screening for ionic liquids dissolving (ligno-)cellulose. Bioresour Technol 100:2580–2587. doi:10.1016/j.biortech.2008.11.052
Zheng Y, Zhao J, Xu FQ, Li YB (2014) Pretreatment of lignocellulosic biomass for enhanced biogas production. Progr Energy Combust Sci 42:35–53
Zhou LL, Wu TH, Wu Y (2012) Degradation and conversion of cellulose in ionic liquids. Prog Chem 24:1533–1543
Acknowledgments
The authors are grateful to the financial support from the Program of International S&T Cooperation of China (2015DFG31860), and the Fundamental Research Funds from the Central Universities (2015ZCQ-CL-02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, JH., Xu, JK., Huang, PL. et al. Effect of alkaline pretreatment on the preparation of regenerated lignocellulose fibers from bamboo stem. Cellulose 23, 2727–2739 (2016). https://doi.org/10.1007/s10570-016-0983-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-0983-1