Abstract
Cellulose fiber charge is a significant parameter for porous cellulose fibers, and strongly affects the swelling ability of cellulose fibers and the properties of cellulose-based materials as well. Actually, it includes surface charge and inner charge. The surface charge is mentioned often in papermaking wet-end chemistry, however, the inner charge or the total charge is paid less attention to. In this study, the cationic polydiallyldimethyl ammonium chloride (poly-DADMAC) with different molecular weight (Mw) was applied for the accessibility evaluation to the cellulose fiber charges by using polyelectrolyte adsorption technique. Results showed that higher fiber charge was detected by lower Mw poly-DADMAC (7.5–15 kDa) due to its highly efficient penetration into the fiber cell walls and neutralization with inner charges, while lower fiber charge was obtained by using higher Mw poly-DADMAC (higher than 100 kDa) because of its adsorption onto fiber surface. As a consequence, high-Mw poly-DADMAC was used to determine the surface charge of cellulose fibers, and low-Mw poly-DADMAC could be used to measure the total charge under the saturated adsorption and low ionic strength (or salts concentration). This was confirmed by SEM–EDS analysis. The low-Mw poly-DADMAC adsorption had a good agreement with conventional conductometric titration, and a linear regression equation with slope of 1.03 and regression coefficient of 0.99 was obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose fiber, refined from natural plants, is a porous compound with mainly cellulose and other substances, such as hemicelluloses and lignin etc. (Maloney and Paulapuro 1999; Fahlén and Salmén 2005; Kimura et al. 2014). In recent years, cellulose fiber, as one kind of biomass raw material, has obtained more and more application in various fields because of its abundance, low cost and good properties (Shen et al. 2011; González et al. 2014; Li et al. 2015; Miao et al. 2015).
Fiber charge is a significant characteristic of cellulose fibers, which strongly affects the post-processing of cellulose fibers, such as enzymatic modification, and the properties of cellulose-based end-use products. The cellulose fiber charge correlates to the amount of anionic functional groups. The amount of these functional groups, including carboxyl, sulfonic acid groups and ionizable hydroxyl groups, varies with cellulose fiber origin and chemical treatments, such as pulping and bleaching processes (Ni et al. 2011; Bhardwaj et al. 2007; He et al. 2005). Generally, the charge density is higher for non-wood cellulose fibers than that for most of wood cellulose fibers, and more charged groups have been found in hardwood fibers than that in softwood fibers because of relatively higher content of glucuronoxylans in hardwood fibers that constraint more carboxylic groups (Banavath et al. 2011; Sjöström 1989). Chemical treatments, which are along with oxidation and degradation of hemicelluloses and lignin in fibers, always lead to the variation in charge density (He et al. 2005; Horvath and Lindström 2007; Miao et al. 2014b).
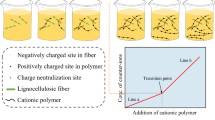
Actually, the charge of cellulose fiber includes two parts, surface charge and inner charge, and the total amount of both charges is defined as the cellulose fiber total charge. Some previous studies (Zhang et al. 1994; Bhardwaj et al. 2007; Sim et al. 2014) reported the significant effects of fiber charge on various properties of cellulose fibers and cellulose-based products. Fiber surface charge affects the bonding strength of cellulose fiber network, while the total charge was highly relevant to fiber hydrophilicity and swelling ability. Surface charge and charge ratio (surface charge/total charge) are important for the adsorption of polyelectrolytes and consequently have a significant influence on the application scopes and chemistry environment of cellulose fibers (Sezaki et al. 2006; Ni et al. 2011).
Various methods have been developed to measure the cellulose fiber charge, including conductometric titration and potentiometric titration used for total charge measurement (Bhardwaj et al. 2004; Fras et al. 2004, 2005), and X-ray photoelectron spectroscopy (XPS), attenuated total reflection flourier transformed infrared spectroscopy (ATR-FTIR) and different sorption methods used for analysis of surface anionic groups (Fardim et al. 2005). The polyelectrolyte adsorption technique using high Mw poly-DADMAC was normally applied to quantitative measurement of fiber surface charge because these polymers could not penetrate into porous fiber (Wågberg et al. 1989). The effect of porous structure in cellulose fibers on polymers accessibility was involved in some findings (Hubbe et al. 2007; Fatehi and Xiao 2008; Miao et al. 2014a). Bhardwaj et al. (2007) summarized that the large difference in charge ratios (varied from 0.17 to 0.8) strongly depended on fiber type and molecular weight of polymer used. Horvath et al. (2006) believed that the polyelectrolyte titration method was a powerful technique for cellulose fiber surface charge determination on a condition that the adsorbed polyelectrolyte had a sufficiently high Mw as well as the titration was carried out in a solution with low ionic strength. The charge stoichiometry and kinetics upon adsorption of cationic polyelectrolyte onto cellulose fibers were studied (Wågberg and Hägglund 2001; Horvath et al. 2006). Although many previous researches concentrated on the adsorption of high Mw polyelectrolyte on cellulose fiber, there are limited literatures concerning about the relation of poly-DADMAC (especially the low Mw poly-DADMAC) accessibility to cellulose fiber and fiber detected charge.
In the study, poly-DADMACs with wide range of molecular weight were used for polyelectrolyte adsorption onto cellulose fibers. The accessibilities of the polymers were analyzed by using SEM–EDS analysis. The low-Mw poly-DADMAC was determined for the total fiber charge measurement, although the high-Mw poly-DADMAC was assured that it could be used for the fiber surface charge measurement. The adsorption time and salts concentration were investigated respectively to improve this process. The major objective was to develop a systematic technique based on polyelectrolyte adsorption for the determination of not only the surface charge but also the total charge of cellulose fibers.
Experimental
Chemicals
A series of commercial poly-DADMACs, obtained from a Chemical Company in Shandong province in China, were used as cationic polyelectrolytes, and potassium polyvinylsulfate (PVSK, Mw = 240 kDa, provided by Wako Pure Chem. Ltd., Japan) with reagent grade was used as anionic titrant. Other chemicals with analytical grades, including NaOH, NaHCO3, NaCl and HCl, were got from the local chemical companies in Tianjin of China.
The Mw and Mw distribution of poly-DADMAC were determined by size-exclusion chromatography (SEC, Waters 1515, USA) at a mobile phase 0.5 M NaCl, flow-rate 0.5 ml/min and temperature 25 °C (Kokufuta and Takahashi 1986). The charge density of poly-DADMAC was determined by direct polyelectrolyte titration with PVSK. The characteristics of poly-DADMACs are listed in Table 1.
Materials
Four different cellulose fibers used were poplar alkaline peroxide mechanical pulp performed with refiner-chemical preconditioning treatment (P-RC APMP) and poplar chemi-thermal mechanical pulp (CTMP) used as lignin-enriched high yield pulp fibers, poplar bleached kraft pulp (HBKP) and softwood bleached kraft pulp (SBKP) used as cellulose-based chemical pulp fibers, provided by commercial pulp mills in China.
P-RC APMP fiber was classified by using Bauer–McNett fiber classifier (TMI 8901-5, USA) according to TAPPI standard method T233 cm-95 (1995), and the fiber fraction P50/R100 was used in this study. The P-RC APMP fibers with different carboxyl group contents were obtained by using TEMPO-mediated oxidation (Ma et al. 2012).
Charge determination
Firstly, the cellulose fiber (0.5 g) was soaked with overdosed 0.01 M HCl to remove metal ions under the conditions of 1 % fiber consistency, pH value 2, and 30-min stirring. Then, the fiber was filtered by using Büchner funnel and washed several times with deionized water until the conductivity of filtrate was below 5 μS/cm. This was defined as the protonated form of the cellulose fiber. Subsequently, the fiber with protonated form was soaked in 10−3 M NaHCO3 solution under a condition of 1 % fiber consistency, pH value 9, and 10-min stirring. Then, the fiber was filtered in a Büchner funnel and washed with deionized water until the conductivity of filtrate was below 5 μS/cm. This was defined as the sodium form of cellulose fiber.
Polyelectrolyte adsorption
The cellulose fiber (0.5 g) in its sodium form was dispersed in excess 10−3 N poly-DADMAC solution with the pH value about 5, temperature 25 °C. The magnetic stirring was to keep the fiber suspension uniform. For long adsorption time, the cellulose fiber was filtered with a pre-weighed quantitative filter paper and dried in oven in order to record the dry weight. The filtrate was collected for polyelectrolyte titration by using a MÜTEK particle charge detector (PCD-03, Germany). 10 ml of the filtrate was pipetted into the cell of PCD-03 and titrated with 10−3 N PVSK until the streaming potential reached 0 mV. Blank value was determined by titrating 10 ml of the poly-DADMAC solution. The polyelectrolyte adsorbed mass or detected charge of cellulose fiber was calculated according to the following formula, Eq. (1).
where q is the detected charge of cellulose fiber (mmol/kg), V b is blank value (ml), V p is volume of titrant used for filtrate (ml), c is concentration of PVSK titrant (mol/l) and w is weight of cellulose fiber (g).
To study the desorption of polyelectrolyte by adding salts, the fiber pre-treated with polyelectrolyte adsorption was withdrawn from the solution and then immersed in solution of different salts concentration. After 12 h, the fiber was filtered with a pre-weighed quantitative filter paper and dried in oven in order to record the dry weight.
The residual polyelectrolyte adsorbed on fiber was determined by polyelectrolyte titration. The filtrate and blank solution with high salts concentration were diluted for polyelectrolyte titration by using PCD-03.
Conductometric titration
About 0.5 g protonated cellulose fiber was dispersed in 100 ml 10−3 M NaCl solution, followed by the addition of 0.5 ml of 0.05 M HCl. Then, the titration was performed with 0.05 M NaOH at 25 °C by using a METTLER TOLEDO conductivity meter (FE30, Switzerland) in a nitrogen environment. During the titration, the suspension was stirred with a magnetic stirrer. The volume of NaOH added and conductivity of suspension was recorded correspondingly for curve plotting. Finally, the suspension was filtered with a pre-weighed quantitative filter paper and dried in oven in order to record the dry weight of cellulose fiber. The total charge was determined using the volume of NaOH required to reach the second inflection point from the first inflection point of curve.
SEM–EDS measurement
Firstly, the cellulose fiber sample was soaked in an overdosed poly-DADMAC solution for overnight, and then filtered by a Büchner funnel without washing. The filter disc was dried in a vacuum drying chamber and then refrigerated with embedding media (mainly the mixture of polyvinyl alcohol, polyethylene glycol and potassium formate). The freeze-dried fiber was cut into thin slices (−5 μm) to obtain the cross-section of single fiber by an ultramicrotome (Shandon Cryotome FE/FSE, Thermo Scientific, USA). The prepared sample was coated with gold film prior for the observation of scanning electron microscope (SEM, LEO 1530 VP, Germany) combined with Energy Dispersive Spectrometer (EDS, Phoenix DX60S, USA).
BET analysis
The pore size of cellulose fiber was determined by Brunauer–Emmett–Teller (BET), according to Kimura et al. (2014). The samples were sequentially solvent-exchanged by using ethanol to replace water and then vacuum dried at room temperature in a vacuum oven. Before nitrogen adsorption measurement, the samples were heated at 105 °C for 8 h. Nitrogen adsorption/desorption isotherms were measured by a Nova 2000e with NovaWin2 data processing (Quantachrome Instruments, USA).
Results and discussion
Effect of molecular weight of poly-DADMAC on the detected charge of cellulose fibers
A series of poly-DADMACs with different molecular weights were used for the polyelectrolyte adsorption onto cellulose fiber. To simplify the evaluation, the classified P-RC APMP fiber fraction (P50/R100), with total charge of 158 mmol/kg determined by conductometric titration, was used, and the proposed time for adsorption was 2 h.
The results of fiber charges detected by different molecular weights of poly-DADMAC are shown in Fig. 1. Fiber detected charge strongly depended on the molecular weight of polyelectrolyte. When the molecular weight decreased from 100 to 8.5 kDa, the fiber detected charge increased from 29 to 107 mmol/kg correspondingly. It seemed that lower Mw poly-DADMAC used for adsorption resulted in a higher detected fiber charge. Similar results have been reported by Horvath et al. (2006) when using bulk carboxymethylated cellulose fiber, as well as reviewed on different cellulose fibers by Bhardwaj et al. (2007). However, the fiber detected charge was nearly unchanged when the Mw varied from 600 to 100 kDa, which implied a similar accessibility of these polymers with relatively high Mw to the fiber cell wall.
The accessibilities of polymers to fiber cell wall can be affected by fiber pores and polymer dimensions (Fatehi et al. 2009, 2011; Horvath et al. 2008). The average pore diameter of this fiber determined by BET was 11.4 nm, and pore size distribution was mainly in 8–20 nm, similar with the report of Kimura et al. (2014). It should be stated that the cellulose fiber was well dried before BET measurement. Fiber drying would lead to the collapse of fiber pores, along with the reducing of pore size (Park et al. 2006). The pore size in an aqueous solution might be higher. Andreasson et al. (2003) reported the average fiber pore radius determined by nuclear magnetic resonance (NMR) measurement was in range of 10–25 nm. Findings of Fatehi et al. (2011) reported that the average radius of assumed cylindrical pores determined by solute exclusion technique was 14.4 nm. By contrast, as obtained earlier by Burkhardt et al. (1987), the radius of gyration was about 8.5–12 nm for poly-DADMAC (Mw = 7.5–15 kDa), versus 24.4–38.1 nm for poly-DADMAC (Mw = 100–280 kDa) respectively in 1 M NaCl solution (Wågberg and Hägglund 2001; Hubbe et al. 2011). If the effect of salt concentration on polymer configuration and the interaction between polymer and fiber were not considered, we could conclude that the low Mw polyelectrolyte (Mw < 15 kDa) with smaller size could penetrate into most of fiber pores, and high Mw polyelectrolyte (Mw > 100 kDa) would be effectively excluded by pore size. In fact, when considering that the polymer would extend in a much diluted solution and the electrostatic interaction between polymers and fiber would reinforce the penetration (Horvath et al. 2006, 2008; Hubbe et al. 2010), this conclusion should be careful. However, the leveling-off value in high Mw poly-DADMAC (Mw > 100 kDa) adsorption reasonably suggested that these polymers only adsorbed on fiber surface regions by electrostatic interactions and could not reach the fiber inner charge sites through the pores. Therefore, higher Mw poly-DADMAC were chosen as probes for fiber surface charge measurement by using polyelectrolyte adsorption, as shown in Fig. 2. As for the low Mw poly-DADMAC (Mw = 7.5–15 kDa) adsorption, the gradually increased detected charge obtained was attributed to the penetration of these polymers into pores (Hubbe et al. 2007; Fatehi et al. 2009, 2011). The detected charge ratio of 0.68 appeared to confirm that this low Mw polymer could penetrate into most of pores in 2 h.
Accessibility analysis by using SEM–EDS
As known, there is no any nitrogen element in the original cellulose fiber cell wall and many nitrogen elements existing in the poly-DADMAC structure, so that the accessibility of the polymers to fiber cell wall can be analyzed based on the local nitrogen element information tested by SEM–EDS technique. The SEM–EDS image of a single treated P-RC APMP P50/R100 fiber cross section is shown in Fig. 3. The marked “A” represented the fiber surface region, the marked “B” represented the interior region of fiber cell wall and “C” was the inner surface of fiber cell wall, close to the lumen.
It can be seen in Fig. 3 that the nitrogen element was detected in three regions of A, B and C in the cellulose fibers treated by low-Mw poly-DADMAC (Mw = 7.5–15 kDa), whereas it was only found in surface area for cellulose fibers treated by high-Mw poly-DADMAC (460–600 kDa). This strongly confirmed the fact that the low-Mw polymer could penetrate into cellulose fiber pores and have a fully accessibility to the fiber cell wall, while the high-Mw polymer was excluded by pores and consequently only adsorbed on the fiber surface.
Time for saturated adsorption
The extension of contact time reinforces the penetration of polyelectrolytes to porous cellulose fibers and leads to the saturated adsorption (Wågberg and Hägglund 2001; Sezaki et al. 2006; Fatehi et al. 2011). It was of great interest to evaluate the extent of the penetration for low-Mw poly-DADMAC. The substantial increasing values of detected charge measured with these lower Mw polyelectrolytes (seen in Fig. 1) implicated the state of unsaturated adsorption, that is, the low-Mw poly-DADMAC was not fully accessible to all of cellulose fiber charges in 2 h.
Adsorption contact times for the low-Mw and high-Mw poly-DADMAC on aspen P-RC APMP (high-yield pulp fiber) and HBKP (chemical pulp fiber) were investigated, respectively. As shown in Fig. 4, the adsorbed polymers initially increased over the time, and then leveled off at a certain level for both P-RC APMP and HBKP, indicating that the adsorbed mass gradually reached plateau. The cationic poly-DADMAC with low molecular weight (Mw = 7.5–15 kDa) showed more adsorbed mass consistently than that with high molecular weight, since low-Mw poly-DADMAC could penetrate and neutralize the inner fiber charges, as discussed above. As the contact time was extended to more than 24 h, the trend changed a little whatever the Mw of poly-DADMAC was low or high. This is in agreement with that summary of Horvath et al. (2008).
Whereas, the different contact times for saturated adsorption process were obtained with the variation of poly-DADMAC molecular weight and cellulose fiber type. For P-RC APMP, the leveling-off value for high-Mw poly-DADMAC was about 2 h, much shorter than that (9 h) for low-Mw poly-DADMAC, and similar trends could be obtained for HBKP. As expected, the high-Mw poly-DADMAC with large dimension only adsorbed onto the cellulose fiber surface, hence, less contact time was needed (Hubbe et al. 2011). In comparison, more contact time was needed for the diffusion of low-Mw poly-DADMAC into the fiber cell walls. It was obvious that the adsorption rate of these low-Mw polymers for HBKP was higher than that for P-RC APMP. This phenomenon was presumably due to difference in pore size distribution of two cellulose fibers. Significant changes to existing pores in cellulose fiber cell walls and the formation of new pores could be involved in the delignification of kraft pulping process and pulp refining (Berthold and Salmén 1997; Maloney and Paulapuro 1999; Fahlén and Salmén 2005). In this study, the mesopore size of P-RC APMP and HBKP determined by BET was 11.4 and 14.8 nm, respectively. Related findings of Hubbe et al. (2011) reported the faster diffusion of poly-DADMAC to large pores than that to small pores of silica. The effect of pore size of cellulose fibers on polyelectrolyte adsorption kinetics needs to be further investigated.
Effect of salts concentration on adsorption and desorption
The interactions of low and high Mw poly-DADMAC with the cellulose fiber were investigated at different salts (NaCl and CaCl2) concentration respectively. Time for adsorption was fixed at 12 h. As shown in Fig. 5, adsorbed mass of high and low Mw poly-DADMAC appeared similar variation with the addition of salts, although divalent CaCl2 showed more sensitive effect on polyelectrolyte adsorption.
The adsorbed mass of poly-DADMAC increased first with the salts addition in Fig. 5, mainly due to the adsorption and penetration of the coiled poly-DADMAC chains onto the surface or into the cell wall of cellulose fiber, whatever the poly-DADMAC Mw is high or low. This phenomenon is caused by the screening effect of high ionic strength, and deviates from 1:1 stoichiometry (Kokufuta and Takahashi 1986; Horvath et al. 2006; Hubbe et al. 2007; Horvath et al. 2008). With the continuous increase of ionic strength, the adsorbed mass of two poly-DADMACs decreased dramatically. This is mainly because of the reduced electrostatic attraction between polyelectrolyte and substance surface at a high salts concentration (Van de Steeg et al. 1992). This phenomenon was in accordance with the previous studies of Lindström and Wågberg (1983) using cationic polyacrylamides. Therefore, the process of polyelectrolyte adsorption must be conducted with a pretty low ionic strength, avoiding the deviation from the stoichiometry.
Also, we found that the pre-adsorbed poly-DADMAC (regardless of low and high Mw polymers) on cellulose would be desorbed at a very high salts concentration, as seen in Fig. 6. With the increase of ionic strength, the interaction between polyelectrolyte segments and fiber surface became unstable since electrostatic screening. The pre-adsorbed polyelectrolyte was replaced by small ions (Van de Steeg et al. 1992). The decrease of low-Mw polyelectrolyte adsorption also indicated that the poly-DADMAC distributed in fiber inner regions could exude from fiber pores.
Comparison with conductometric titration
For a monolayer of adsorbed polyelectrolyte on cellulose fibers, it was verified that a high stoichiometry (above 0.9) was for both high and low Mw polyelectrolytes adsorption on cellulose fibers (Wågberg 2000). Recent study (Horvath et al. 2006) clarified the rational conformity in ESCA analysis between these two polyelectrolytes and the similar thickness of adsorbed layer for both polyelectrolytes. These conclusions essentially also proved the well applicability of these polyelectrolytes used for charge analysis of cellulose fiber. It should be stated that enough contact time (more than 9 h in this study) must be kept for low-Mw polyelectrolyte fully accessible to fiber wall, when this polymer was used for measuring fiber total charge. On the contrary, relatively less time is required for surface charge measurements because high-Mw polyelectrolyte only need to adsorb onto the cellulose fiber surface. Besides, the polymer adsorption process must conduct in a much diluted solution.
Conductometric titration, a conventional method based on the conductivity technology (Fras et al. 2004), was widely used for cellulose fiber total charge determination for its convenient operation. The fiber charges obtained from polyelectrolyte adsorption technique were used for comparison with the charges measured by conductometric titration to verify the reliability.
Table 2 shows the values of fiber charge analyzed for the four pulp fibers. The high yield pulp fibers (P-RC APMP and CTMP) had a higher total charge than chemical pulp fibers (HBKP and SBKP), which was related to the high retention of hemicelluloses and lignin containing many charged groups in high yield pulps (Buchert et al. 1995; He et al. 2005). Meanwhile, hardwood cellulose fiber had a higher total charge than softwood cellulose fiber for its higher content of glucuronoxylans that combined numerous carboxylic groups (Buchert et al. 1995; Ni et al. 2011). The total charges obtained from polyelectrolyte adsorption technique were similar to the results detected by conductometric titration. The deviation (in range of 0.08) between both methods was negligible. The corresponding charge ratios were 0.26, 0.21, 0.34 and 0.4 based on conductometric titration, and 0.24, 0.21, 0.32, 0.38 based on polyelectrolyte titration, respectively for P-RC APMP, CTMP, HBKP and SBKP.
The P-RC APMP fibers with different carboxyl group contents, obtained by TEMPO-mediated oxidation, were used for total charge measurement and further correlation analysis of both methods. It can be seen in Fig. 7 that the polyelectrolyte adsorption charges were in a strong agreement with the conductometric titration charges. The linear relationship, with slope of 1.03 and regression coefficient of 0.99, was obtained for the fibers with a wide difference in charge density, which substantially gave a strong credibility to the idea that the low-Mw poly-DADMAC could be applied to accurate analysis of cellulose fiber total charge by using polyelectrolyte adsorption technique.
Conclusions
The fiber charge detected by polyelectrolyte adsorption was high relevant to the molecular weight of poly-DADMAC used. Low-Mw polymers resulted in high detected charge, whereas high-Mw polymers led to low detected charge. The results could be effectively confirmed by SEM–EDS images that low-Mw polymers could penetrate into the cellulose fiber cell walls while high-Mw polymers only adsorbed on the fiber surface. On a condition that the ionic strength was much low and the contact time was long enough, the low-Mw poly-DADMAC (7.5–15 kDa) could be used for fiber total charge measurement and the high-Mw poly-DADMAC (Mw > 100 kDa) could be used for fiber surface charge measurement. The fiber total charge measured by polyelectrolyte adsorption technique with low-Mw poly-DADMAC showed a strong agreement to the charge detected by conductometric titration.
References
Andreasson B, Forsström J, Wågberg L (2003) The porous structure of pulp fibers with different yields and its influence on paper strength. Cellulose 10:111–123
Banavath HN, Bhardwaj NK, Ray AK (2011) A comparative study of the effect of refining on charge of various pulps. Bioresour Technol 102(6):4544–4551
Berthold J, Salmén L (1997) Effects of mechanical and chemical treatments on the pore-size distribution in wood pulps examined by inverse size-exclusion chromatography. J Pulp Pap Sci 23(6):J245–J253
Bhardwaj NK, Duong TD, Nguyen KL (2004) Pulp charge determination by different methods: effect of beating/refining. Colloids Surf A Physicochem Eng Asp 236(1):39–44
Bhardwaj NK, Hoang V, Nguyen KL (2007) A comparative study of the effect of refining on physical and electrokinetic properties of various cellulosic fibres. Bioresour Technol 98(8):1647–1654
Buchert J, Teleman A, Harjunpaa V, Tenkanen M, Viikari L, Vuorinen T (1995) Effect of cooking and bleaching on the structure of xylan in conventional pine kraft pulp. Tappi J 78:125–128
Burkhardt CW, McCarthy KJ, Parazak DP (1987) solution properties of poly (dimethyldiallylammonium chloride). J Polym Sci 25:209–213
Fahlén J, Salmén L (2005) Pore and matrix distribution in the fiber wall revealed by atomic force microscopy and image analysis. Biomacromolecules 6(1):433–438
Fardim P, Moreno T, Holmbom B (2005) Anionic groups on cellulosic fiber surfaces investigated by XPS, FTIR-ATR, and different sorption methods. J Colloid Interface Sci 290(2):383–391
Fatehi P, Xiao H (2008) Adsorption characteristics of cationic-modified poly (vinyl alcohol) on cellulose fibers—a qualitative analysis. Colloids Surf A Physicochem Eng Asp 327(1):127–133
Fatehi P, MacMillan B, Ziaee Z, Xiao H (2009) Qualitative characterization of the diffusion of cationic-modified PVA into the cellulose fiber pores. Colloids Surf A Physicochem Eng Asp 290(2):383–391
Fatehi P, Xiao H, van de Ven TG (2011) Quantitative analysis of cationic poly (vinyl alcohol) diffusion into the hairy structure of cellulose fiber pores: charge density effect. Langmuir 27(22):13489–13496
Fras L, Laine J, Stenius P, Stana-Kleinschek K, Ribitsch V, Doleček V (2004) Determination of dissociable groups in natural and regenerated cellulose fibers by different titration methods. J Appl Polym Sci 92(5):3186–3195
Fras L, Johansson LS, Stenius P, Laine J, Stana-Kleinschek K, Ribitsch V (2005) Analysis of the oxidation of cellulose fibres by titration and XPS. Colloids Surf A Physicochem Eng Asp 260(1):101–108
González I, Alcalà M, Chinga-Carrasco G, Vilaseca F, Boufi S, Mutjé P (2014) From paper to nanopaper: evolution of mechanical and physical properties. Cellulose 21(4):2599–2609
He Z, Ni Y, Zhang E (2005) Further understanding on the cationic demand of dissolved substances during peroxide bleaching of a spruce TMP. J Wood Chem Technol 24(2):153–168
Horvath AE, Lindström T (2007) Indirect polyelectrolyte titration of cellulosic fibers. Surface and bulk charges of cellulosic fibers. Nord Pulp Pap Res J 22(1):87–92
Horvath AE, Lindström T, Laine J (2006) On the indirect polyelectrolyte titration of cellulosic fibers. Conditions for charge stoichiometry and comparison with ESCA. Langmuir 22(2):824–830
Horvath AT, Horvath AE, Lindstrom T, Wågberg L (2008) Diffusion of cationic polyelectrolytes into cellulosic fibers. Langmuir 24(19):10797–10806
Hubbe MA, Rojas OJ, Lucia LA, Jung TM (2007) Consequences of the nanoporosity of cellulosic fibers on their streaming potential and their interactions with cationic polyelectrolytes. Cellulose 14(6):655–671
Hubbe MA, Wu N, Rojas OJ, Park S (2010) Permeation of a cationic polyelectrolyte into mesoporous silica. Part 2. Effects of time and pore size on streaming potential. Colloids Surf A Physicochem Eng Asp 364(1):7–15
Hubbe MA, Wu N, Rojas OJ, Park S (2011) Permeation of a cationic polyelectrolyte into mesoporous silica: Part 3. Using adsorption isotherms to elucidate streaming potential results. Colloids Surf A Physicochem Eng Asp 381(1):1–6
Kimura M, Qi ZD, Fukuzumi H, Kuga S, Isogai A (2014) Mesoporous structures in never-dried softwood cellulose fibers investigated by nitrogen adsorption. Cellulose 21(5):3193–3201
Kokufuta E, Takahashi K (1986) Adsorption of poly (diallyldimethylammonium chloride) on colloid silica from water and salt solution. Macromolecules 19(2):351–354
Li MC, Wu Q, Song K, Qing Y, Wu Y (2015) Cellulose nanoparticles as modifiers for rheology and fluid loss in bentonite water-based fluids. ACS Appl Mater Interface 7(8):5006–5016
Lindström T, Wågberg L (1983) Effects of pH and electrolyte concentration on the adsorption of cationic polyacrylamides on cellulose. Tappi J 66(6):83–85
Ma P, Fu S, Zhai H, Law K, Daneault C (2012) Influence of TEMPO-mediated oxidation on the lignin of thermomechanical pulp. Bioresour Technol 118:607–610
Maloney TC, Paulapuro H (1999) The formation of pores in the cell wall. Pulp Pap Sci 25(12):430–436
Miao Q, Chen L, Huang L, Tian C, Zheng L, Ni Y (2014a) A process for enhancing the accessibility and reactivity of hardwood kraft-based dissolving pulp for viscose rayon production by cellulase treatment. Bioresour Technol 154:109–113
Miao Q, Zhong G, Qin M, Chen L, Huang L (2014b) Influence of alkaline treatment and alkaline peroxide bleaching of aspen chemithermomechanical pulp on dissolved and colloidal substances. Ind Eng Chem Res 53(6):2544–2548
Miao Q, Tian C, Chen L, Huang L, Zheng L, Ni Y (2015) Combined mechanical and enzymatic treatments for improving the Fock reactivity of hardwood kraft-based dissolving pulp. Cellulose 22:803–809
Ni Y, He Z, Zhang H, Zhou Y (2011) Characteristics of high yield pulp and its effect on some typical wet-end issues. J Biobased Mater Bio 5(2):181–186
Park S, Venditti R, Jameel H, Pawlak J (2006) Changes in pore size distribution during the drying of cellulose fibers as measured by differential scanning calorimetry. Carbohydr Polym 66(1):97–103
Sezaki T, Hubbe MA, Heitmann JA, Argyropoulos DS (2006) Colloidal effects of acrylamide polyampholytes: part 2: adsorption onto cellulosic fibers. Colloids Surf A Physicochem Eng Asp 289(1):89–95
Shen J, Song Z, Qian X, Ni Y (2011) A review on use of fillers in cellulosic paper for functional applications. Ind Eng Chem Res 50(2):661–666
Sim G, Alam MN, Godbout L, van de Ven T (2014) Structure of swollen carboxylated cellulose fibers. Cellulose 21(6):4595–4606
Sjöström E (1989) The origin of charge on cellulosic fibres. Nord Pulp Pap Res J 4(2):90–93
Van de Steeg HGM, Cohen Stuart MA, De Keizer A, Bijsterbosch BH (1992) Polyelectrolyte adsorption: a subtle balance of forces. Langmuir 8(10):2538–2546
Wågberg L (2000) Polyelectrolyte adsorption onto cellulose fibres—a review. Nord Pulp Pap Res J 15(5):586–597
Wågberg L, Hägglund R (2001) Kinetics of polyelectrolyte adsorption on cellulosic fibers. Langmuir 17(4):1096–1103
Wågberg L, Odberg L, Glad-Nordmark G (1989) Charge determination of porous substrates by polyelectrolyte adsorption. Part 1. Carboxymethylated, bleached cellulosic fibers. Nord Pulp Pap Res J 2:71–76
Zhang Y, Sjögren B, Engstrand P, Htun M (1994) Determination of charged groups in mechanical pulp fibres and their influence on pulp properties. J Wood Chem Technol 14(1):83–102
Acknowledgments
The authors acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 31370577).
Author information
Authors and Affiliations
Corresponding author
Additional information
A small part of this paper was reported as an extended abstract in the Proceedings of the 18th ISWFPC (International Symposium on Wood, Fiber and Pulping Chemistry, Vienna, September 9–11, 2015).
Rights and permissions
About this article
Cite this article
Zhang, H., Zhao, C., Li, Z. et al. The fiber charge measurement depending on the poly-DADMAC accessibility to cellulose fibers. Cellulose 23, 163–173 (2016). https://doi.org/10.1007/s10570-015-0793-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0793-x