Abstract
New organic-inorganic mesoporous hybrid materials containing terbium complexes covalently attached to mesoporous silica SBA-15 have been successfully prepared. The mesoporous silica SBA-15 was modified with 3,4,5-tri hydroxyphenyl acetic acid ligand and then used to fabricate the lanthanide-based mesoporous material SBA-15@3,4,5-tri hydroxyphenyl acetic@ Tb. The mesoporous material was characterized by Fourier transforms infrared (FTIR) spectra, powder X-ray diffraction (XRD), and scanning electron microscopy (SEM). The results show that the 3,4,5-tri hydroxyphenyl acetic acid ligand and Tb ions are attached to the SBA-15 host. The catalysts were tested in the synthesis of 5-substituted 1H-tetrazoles. This catalyst is an efficient catalyst for [3 + 2] cycloaddition with NaN3 to prepare 5-substituted 1H-tetrazoles. The catalyst was recycled for up to six cycles without significant loss of activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tetrazoles have received considerable attention in different applications. The presence of tetrazole part in a drug molecule improves cellular penetration and oral bioavailability. Examples of the applications of tetrazoles include their use in special explosives, in pharmaceuticals, and as precursors of various nitrogen-containing heterocyclic compounds such as imidoylazides [1,2,3,4]. The applications of tetrazoles have made chemists try to synthesize tetrazoles more easily and efficiently.
Heterogeneous catalysts are widely used in the industrial production of a wide range of bulk and fine chemicals because they are easily separated from the reaction mixture and can be reused in successive cycles [5,6,7,8,9,10,11]. The reaction occurs at the interface between the reactants and the solid catalyst that this is a major limitation of heterogeneous compared to homogeneous catalysts. Homogeneous catalysts are usually more active due to better contact between the catalytic sites and the reactants. Many research efforts have been aimed at increasing the activity of heterogeneous catalysts by increasing their specific surface area [11,12,13]. Reducing the size of catalyst particles to the nanoscale to increase their surface-to-volume ratio is a way to increase the activity of heterogeneous catalysts [14].
Mesoporous silica materials have attracted considerable interest, covering a wide range of applications in the field of catalysis and separation [11, 15, 16]. Pure silica SBA-15 was limited to being used in various types of catalytic reactions due to its neutral surface and little acidic center. The average pore size and specific surface area of pure silica SBA-15 can be changed, and its catalytic performance can be increased by attaching transition metals to it.
In this contribution, we try to address this issue by designing the novel heterogeneous Catalyst comprising terbium (III) complexes immobilized on mesoporous SBA-15 that are an extremely active catalyst for the synthesis of 5-substituted tetrazoles. To the best of our knowledge, terbium, as an element in the lanthanide series, functionalized SBA-15 for the synthesis of 5-substituted 1Htetrazoles has been rarely reported. Rare-earth-metal complexes have a great interest because of their unique properties such as low toxicity, rich and diverse coordination chemistry, high reactivity, and wide applications in various fields. In this work, novel terbium (III) complexes immobilized in SBA-15 were synthesized via post-functionalization modification of SBA-15 (SBA-15@3,4,5-tri hydroxyphenyl acetic@ Tb) was synthesized and used as a catalyst for the synthesis of 5-substituted 1H-tetrazoles.
2 Experimental
2.1 Synthesis of SBA-15
Pluronic P123 (4.0 g) and 90 mL of HCl (2 M) were introduced to 30 mL of distilled water with stirring at 30 °C for 5 h. After that, tetraethyl orthosilicate (9.04 mL, TEOS) was added to the solution and stirred for 20 h. The product was collected, washed with distilled water, and dried at 60 °C. The SBA-15 was calcinated at 550 °C for 5 h.
2.2 Preparation of SBA-15@3,4,5-tri Hydroxyphenyl Acetic Acid
The SBA-15 (1 g) and 3,4,5-tri hydroxyphenyl acetic acid (1.5 g, M = 184.14 g/mol) were completely dispersed in deionized water (40 mL). The mixture was refluxed for 48 h. After that, the SBA-15@3,4,5-tri hydroxyphenyl acetic acid was collected, washed with water, and dried.
2.3 Preparation of SBA-15@3,4,5-tri Hydroxyphenyl Acetic aci @Tb
The SBA-15@3,4,5-tri hydroxyphenyl acetic acid (1 g) and TbCl3 (2.5 mmol) in ethanol solvent (40 mL) were refluxed for 16 h. After that, the SBA-15@3,4,5-tri hydroxyphenyl acetic acid@Tb was collected, washed several times with ethanol, and dried (Scheme 1).
2.4 General Process for the Synthesis of 1 H- tetrazoles
3 ml of H2O was added to the mixture of nitrile (1 mmol), sodium azide (1.2 mmol, 0.078 g), and catalyst (70 mg) and placed at 80 °C. Progression of the reaction was followed by TLC (ethyl acetate/n-hexane”). After the end of the reaction, the catalyst was filtered and the reaction mixture was treated with ethyl acetate and acidified with HCl (10 mL, 5 M). After that, 2–5 mL of cold water was added and washed several times. Finally, it was dried.
2.5 Selected Spectral Data
(Table 1, Entry 1) 5-(3-Nitrophenyl)-1 H-tetrazole. 1HNMR (400 MHz, DMSO, ppm): δ 7.90 (t, 2 H), 8.39–8.49 (m, 2 H), 8.40–8.49 (s, 1 H.)
(Table 1, Entry 2) 5-(4-Nitrophenyl)-1 H-tetrazole1HNMR (400 MHz, DMSO, ppm): δ 8.29–8.33 (d, 2 H), 8.44–8.46 (d, 2 H).
3 Results and Discussion
3.1 Characterization
The small angle X-ray diffraction patterns of SBA-15@3,4,5-tri hydroxyphenyl acetic acid @Tb sample are illustrated in Fig. 1. The sample displays a strong peak around 2θ = 1.27 °, and weak peaks at 2.22, and 2.9°, corresponding to the diffraction of (100), (110), and (200), respectively [17]. These diffraction peaks are characteristic of typical 2-D hexagonal mesostructured and order of the silica matrix.
The SEM images and EDS spectra of SBA-15@3,4,5-tri hydroxyphenyl acetic acid @Tb sample are illustrated in Figs. 2. It can be seen that the diameters of SBA-15@3,4,5-tri hydroxyphenyl acetic acid @Tb particles were uniform. The presence of Tb, C, O, and Si atoms in the structure of the catalysts were confirmed by EDS spectra of SBA-15@3,4,5-tri hydroxyphenyl acetic acid @Tb.
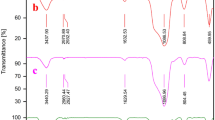
The FT-IR analysis of SBA-15@3,4,5-tri hydroxyphenyl acetic acid and SBA-15@3,4,5-tri hydroxyphenyl acetic acid @Tb are depicted in Fig. 3. The FT-IR spectra of the samples have bands at round 463, 860, and 1090 cm− 1 which are from the bending Si − O−Si vibration, the symmetric, and asymmetric Si − O−Si stretching vibration respectively [18]. The bands that appear at 3400–3500 cm− 1 are a characteristic band of Si–OH and water molecules adsorbed. The band at round 1615 cm− 1 1386 cm− 1 is attributed to stretching vibrations of aromatic C = C, and C–O stretching vibration. The presence of bands at around 2927 cm− 1 and 2868 cm− 1 are characteristic band of C-H stretching vibrations of 3,4,5-trihydroxyphenylacetic acid. The presence of these bands shows that the, 4,5-tri hydroxyphenyl acetic acid has been effectively anchored to SBA-15 support.
The TGA of SBA-15@3,4,5-tri hydroxyphenyl acetic acid @Tb (Fig. 4) shows 8% weight loss at temperatures below 200 °C which corresponds to the vaporization of physiosorbed water and 10% weight loss at 200–600 °C, which corresponds to the release of water formed during the condensation of silanols in the silica framework and the decomposition of organic templates grafting to the SBA-15.
3.2 Catalytic activity
The SBA-15@3,4,5-tri hydroxyphenyl acetic acid @Tb sample was tested for its ability to catalyze the synthesis of 5-substituted 1H-tetrazoles between 4-Chlorobenzonitrile and NaN3. To find the mildest reaction conditions for the synthesis of 5substituted 1Htetrazoles the reaction conditions were standardized by monitoring the effect of solvents, catalyst loading, (Table 2) and temperature (Table 2). Synthesis of tetrazole was carried out using various solvents such as EtOH, DMSO, DMF, acetonitrile, toluene, EtOH/water and water. The dielectric constant of the solvent plays an important role in stabilizing the reaction intermediate [19, 20]. We also tried a reaction in water with the use of green solvents in reaction media the product yield was higher in water than in other solvents. The influence of catalyst loading was studied in terms of product yield. In the absence of a catalyst, no reaction occurred even after 12 h stirring. The yield was isolated in a lower yield (45%) when the reaction was carried out in the presence of 30 mg of catalyst. When the reaction was performed in the presence of 70 mg of the catalyst this product was improved to 88% in a short reaction time. Further, on increasing the catalyst loading up to 100 mg, there was no significant increase in yield. Hence 80 mg of catalyst was considered as an optimum catalyst loading. Among the different temperature ranges tested, it was found that there was a decrease in the yield with the decrease in temperature up to room temperature. The increase in the reaction rate with temperature was expected according to the Arrhenius equation [21]. For this reaction, the best result was obtained at 80 °C. We next examined the scope of the SBA-15@3,4,5-tri hydroxyphenyl acetic acid @Tb a series of nitrile substitutions were used in optimal conditions and the results are shown in Table 1. In these tables, the desired product was obtained in mild conditions and in short reaction times with good yields.
3.3 Recyclability Study
The recovery of the SBA-15@3,4,5-tri hydroxyphenyl acetic acid @Tb was investigated by its use in the synthesis of 1 H-tetrazole under the optimized reaction conditions. 4-chlorobenzonitrile was chosen as a model substrate. The catalyst was recovered easily at the end of the reaction by simple filtration. The recovered catalyst was washed with EtOAc, and dried at 50 °C. Then reused for a similar reaction. Our experiments showed that the catalyst can be recycled over six cycles. In the recycled sample, the catalytic activity did not decrease significantly after six cycles (Fig. 5).
4 Conclusion
New organic-inorganic mesoporous hybrid materials containing terbium complexes covalently attached to mesoporous silica SBA-15 have been successfully prepared. The mesoporous silica SBA-15 was modified with 3,4,5-tri hydroxyphenyl acetic acid ligand and then used to fabricate the lanthanide-based mesoporous material SBA-15@3,4,5-tri hydroxyphenyl acetic@ Tb. The samples display strong peaks attributed to typical 2-D hexagonal mesostructured and order of the silica matrix. The SEM images and EDS spectra of SBA-15@3,4,5-tri hydroxyphenyl acetic acid @Tb sample show the SBA-15@3,4,5-tri hydroxyphenyl acetic acid @Tb particles were uniform. The presence of Tb, C, O, and Si atoms in the structure of the catalysts were confirmed by EDS spectra. The FT-IR band at round 1617 cm− 1 1384 cm− 1 are attributed to stretching vibrations of aromatic C = C, and C–O stretching vibration shows that the ,4,5-tri hydroxyphenyl acetic acid has been effectively anchored to SBA-15 support. The TGA of SBA-15@3,4,5-tri hydroxyphenyl acetic acid @Tb shows the decomposition of organic templates grafting to the SBA-15.
Data Availability
These are contained in the paper.
References
Wittenberger SJ (1994) Org Prep Proced Int 26:499
Duncia JV, Pierce ME, Santella JB (1991) J Org Chem 56:2395
Wittenberger SJ, Donner BG (1993) J Org Chem 58:4139
Curran DP, Hadida S, Kim S-Y (1999) Tetrahedron 55:8997
Corma A, Garcia H (2006) Adv Synth Catal 348:1391
Tamoradi T, Ghorbani-Choghamarani A, Ghadermazi M (2017) New J Chem 41:11714
Karimi B, Rafiee M, Alizadeh S, Vali H (2015) Green Chem 17:991
Molaei S, Ghadermazi M (2020) Appl Organomet Chem 34:e5328
Molaei S, Tamoradi T, Ghadermazi M, Ghorbani-Choghamarani A (2018) Catal Lett 148:1834
Tamoradi T, Taheri A, Vahedi S, Ghadermazi M (2019) Solid State Sci 97:105981
Molaei S, Ghadermazi M (2019) Appl Organomet Chem 33:e4854
Molaei S, Tamoradi T, Ghadermazi M, Ghorbani-Choghamarani A (2018) Micropor Mesopor Mat 272:241
Molaei S, Tamoradi T, Ghadermazi M, Ghorbani-Choghamarani A (2018) Catal Lett 148:1834
Collard X, Li L, Lueangchaichaweng W, Bertrand A, Aprile C, Pescarmona PP (2014) Catal Today 235:184
Molaei S, Ghadermazi M (2020) Solid State Sci 100:106091
Molaei S, Tamoradi T, Ghadermazi M, Ghorbani-Choghamarani A (2018) Polyhedron 156:35
Tamoradi T, Ghadermazi M, Ghorbani-Choghamarani A, Molaei S (2018) Res Chem Intermed 44:4259
Appaturi JN, Johan MR, Ramalingam RJ, Al-Lohedan HA (2018) Micropor Mesopor Mat 256:67
Rath D, Parida KM (2011) Ind Eng Chem Res 50:2839
Gómez-Quero S, Díaz E, Cárdenas-Lizana F, Keane MA (2010) Chem Eng Sci 65:3786
Roy SK, Dutta D, Talukdar AK (2018) Mater Res Bull 103:38
Acknowledgements
The authors are deeply grateful to the University of Kurdistan for the financial support of this research project.
Funding
The authors have no funding for this article.
Author information
Authors and Affiliations
Contributions
MG: Conceptualization, Methodology, Validation, Formal analysis, Resources, Supervision, Project administration, Funding acquisition. SM: Conceptualization, Methodology, Software, Resources, Writing Original Draft, Writing Review and Editing, Visualization, Investigation, Data Curation
Corresponding author
Ethics declarations
Ethical Approval
The authors approve the presentation of the research by following the rules of scientific practice.
Informed Consent
The authors agree to participate in this study.
Consent to Publications
The authors agree to publish this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghadermazi, M., Molaei, S. Synthesis of SBA-15@3,4,5-tri Hydroxyphenyl Acetic@ Tb for the Facile Synthesis of 5-Substituted 1 H-tetrazoles. Catal Surv Asia 27, 139–146 (2023). https://doi.org/10.1007/s10563-022-09375-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10563-022-09375-7