Abstract
The copper benzene-1, 3, 5-tricarboxylate metal–organic framework (CuBTC) was found to be an effective heterogeneous catalyst for the aza-Michael addition reaction of the four types of amines to electron deficient alkenes at room temperature. The catalytic protocol showed high product yields and outstanding chemo selectivity. The cyclic amines (piperidine and pyrrolidine) and aliphatic amines (n-dibutylamine) provided aza-Michael addition with a high yield of product (⁓98%) within shorter reaction period (2 h) at room temperature under mild reaction conditions using CuBTC. However, it was observed that the aza-Michael reaction proceeded more slowly, giving 62% yield of product after 24 h in the case of aromatic amine (aniline) with n-butyl acrylate in the presence of CuBTC under identical reaction conditions. The catalyst could be reused four recycles without losing its initial catalytic activity and selectivity. XRD and SEM analysis further confirmed that the crystallinity of catalyst was retained during the reaction. A reaction mechanism is proposed for the aza-Michael addition reaction over heterogeneous CuBTC catalyst.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Aza-Michael addition reaction is an effective and simplest protocol for the preparation of β–amino carbonyl compounds from amines with electron-deficient alkenes, which was discovered in 1874 [1, 2]. These compounds are one of the most valuable intermediates for the preparation of a wide range of bioactive molecules, antibiotics, peptide analogues, chiral auxiliaries, and other nitrogen-containing compounds [3]. The reaction can also generate a large variety of intermediates viz., sol–gel precursors, coupling agents, reactive diluents, and silicon-based (macro) materials [4].
Traditionally, 1,4-addition of amines with electron-deficient alkenes have been carried out using a stoichiometric amount of Lewis acids, viz., NiCl3.7H2O, [5] LiClO4, [6] Cu(OTf)2, [7] Na2SnO3, CeCl3.7H2O-NaI [8] and BiNO3 [9]. However, the above processes have many drawbacks, such as, difficulty in recovery and reuse of the catalyst, long reaction time, and high temperature, which make the procedure ecologically unacceptable [10, 11]. To overcome the above drawback, ionic liquids or supported ionic liquids have been applied as an efficient catalyst for the formation of α, β–unsaturated compounds; they can be reused without loss of activity, which are time-consuming and costly [12,13,14,15]. Recently, several heterogeneous catalysts for the aza-Michael reaction have been studied, such as Cu-Al hydrotalcite, [16] vinyl sulfones, Amberlyst-15, [17] phosphate impregnated titania, [18] silica-supported aluminum chloride, [19] acidic alumina, [20] Al-KIT5 nano-cage mesoporous, [21] MCM-41 Supported Phenanthrolinium Dibromide, [22] polymer‐shell-encapsulated magnetite nanoparticles, [23] and copper–amine complex supported on mesoporous silica SBA-15 [24]. Further efforts to develop simple and environmentally friendly green protocols are highly desirable.
During the past decade, metal–organic frameworks (MOFs) have gained much attention as potential heterogeneous catalysts for various organic transformation owing to the large surface area, ordered crystalline structure, structural diversity and tunable pore sizes [25,26,27,28,29,30,31,32,33,34]. MOFs have various types of heterogeneous active sites, among them, open-metal sites/coordinatively unsaturated metal sites are one of the most regularly studied ones for catalysis applications [35,36,37]. Recently, Rostamnia et al. reported the catalytic activity of open metal site iron-based MOF (MIL-100(Fe)) for the aza-Michael reaction as an efficient green catalyst [38]. BASF has recently commercialized some important MOFs as adsorbents or catalysts for industrial applications. Among them, CuBTC (copper benzene-1, 3, 5-tricarboxylate) is one of the well-known MOFs for potential adsorbents or catalysts owing to its simple preparation procedure, high yield, excellent textural properties, and stability [35]. This MOF is known as Basolite(TM) C300, which was composed of a cluster of two copper ions with a paddlewheel unit, having unsaturated open metal sites. The material showed good catalytic activity and selectivity for fine chemical preparation by the Lewis acidic or redox properties of the material [35]. Savonnet et al. [39] have reported on the catalytic application of the MOFs as an efficient catalyst for the aza-Michael addition reaction. Nguyen et al. reported that 5 mol% CuBTC was found to be highly active and reusable in selected organic reactions for the aza-Michael addition reaction [40]. In continuation of our work on the MOF-based catalysts for fine chemicals preparation, we report here the possibility and efficiency of CuBTC as a reusable catalyst for the Aza-Michael reaction using a variety of amines, such as cyclic, aliphatic and aromatic with different electron-deficient alkenes at room temperature under mild reaction conditions. In this work, we have expanded the range of substrates to examine the chemo-selectivity of CuBTC to give high product yields and good chemo-selectivity.
2 Experimental
2.1 Materials
Copper(II) nitrate hydrate (98%), 1,3,5-benzenetricarboxylic acid (95%), piperidine (99%), pyrrolidine (99%), dibutylamine (99.5%), aniline (ACS reagent, 99.5%), 4-methylaniline (99.6%), 4-chloroaniline (99%), n-butyl acrylate (99%), methyl acrylate (99%) and n-dodecane (99%) were purchased from Sigma-Aldrich and were used as received.
2.2 Synthesis of Cu(BTC)
The MOF structure was prepared according to our previously published method using the ethanol reflux method [41]. Briefly, copper(II) nitrate hydrate (0.875 g) and trimesic acid (0.42 g) in 25 ml ethanol was heated under reflux with constant stirring for 24 h and cooled to room temperature. The blue precipitated was separated by filtration, washed with water and then with ethanol and dried at 100 ℃ under vacuum for 5 h.
2.3 Characterization
XRD pattern of the sample was obtained using a Rigaku UltimaIV diffractometer with CuKα radiation (λ = 1.54) at 0.2 0 min-1. SEM micrographs were taken on a JEOL (JSM-6490LA) instrument. The N2 adsorption–desorption measurements were measured on a BELsorp Mini (BEL Corporation, Japan) instrument at 77 K. The surface areas were calculated by the BET (Brunauer–Emmett–Teller) method, and the pore volumes and pore diameters were estimated from desorption branch of the isotherm based on BJH (Barrett-Joyner-Halenda) model. Prior to the measurement, the sample was degassed at 150 ℃ under vacuum for 3 h. FTIR spectra were recorded as KBr discs on a IRPrestige 21 (Shimadzu, Japan) spectrometer. Cu content in the reaction product solution was measured by atomic absorption spectroscopy using Perkin-Elmer AAS AAnalyst 200.
2.4 Catalytic Reaction
The aza-Michael addition reaction of amines with alkenes over CuBTC as catalyst was carried out in a round-bottom flask equipped with a condenser. In a typical run, 2 mmol of piperidine, 5 mol% of CuBTC, methanol (4 mL), and 0.88 mmol of n-dodecane as an internal standard were stirred at room temperature for 20 min. n-Butyl acrylate (2 mmol) was added to the mixture with constant stirring. After completion of the reaction, ethyl acetate (5 mL) was added and then the catalyst was filtered off and the material washed with methanol and dried under vacuum for 5 h at 100 ℃, and reused for further reaction. The filtrate was dried over Na2SO4, and the conversion and product selectivity were measured using a GC (Clarus 500, Perkin-Elmer) fitted with a high performance HP-1 capillary column and FID.
A hot filtering experiment of the aza-Michael addition reaction of piperidine and n-butyl acrylate was carried out by separating the CuBTC from the reaction mixture after 60 min of reaction. The filtrate mixture was then stirred for a further 65 min at room temperature. The conversion and selectivity were measured by GC.
3 Results and Discussion
The copper benzene-1, 3, 5-tricarboxylate (CuBTC) metal–organic framework structure has been readily prepared and well characterized [35]. Ahn et al. described the preparation method on a bench scale having good textural properties by the solvothermal method using a 1 L-capacity Pyrex reactor in ethanol medium [41]. The material was characterized by XRD, SEM, N2 adsorption–desorption isotherm and FT-IR. As shown in Figs. 1 and 2, XRD and SEM of present material confirmed that the MOF showed characteristic XRD and morphology as reported previously [35, 41]. N2 adsorption–desorption isotherms and pore size distribution curves are shown in Fig. 3. The material showed a type I pattern [35]. The BET surface area and pore volume were 1304 cm2/g and 0.650 cm3/g, respectively. As shown in the Fig. 3, the pore size distribution curve was centered at 1.5 nm. The CuBTC showed strong bands at 1652, 1450 and 1374 cm−1 in the IR spectrum (Fig. 4a) due to stretching modes of the C-O group, as well as the absence of strong bands in the region 1760–1690 cm−1 assigned to C = O stretching vibration, indicated the deprotonation of –COOH in the trimesic acid upon the reaction with copper cations [40].
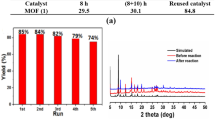
The prepared CuBTC material was tested as a catalyst for the aza-Michael reaction of various amines to electron-deficient alkenes at room temperature. The reaction was carried out using 5 mol% CuBTC under the identical conditions previously reported by Nguyen et al. [40] and the results are listed in Table 1. Using 5 mol% catalyst, the catalyst formed a mono-addition product only in all cases. Initially, blank test experiments were carried out using piperidine with n-butyl acrylate under identical reaction conditions as described in Table 1 in the absence of CuBTC. Piperidine was converted to a product with 9% yield after 125 min. After 125 min, piperidine was converted to mono-addition products with 98% yield at room temperature in the presence of 5 mol% CuBTC under the same reaction conditions (Fig. 5). The influence of reaction time on the catalytic performance is shown in Fig. 6. The product yield gradually increased with increasing reaction time.
To examine the influence of different alkenes, the reaction was performed between piperidine and methyl acrylate as the Michael acceptor, forming a corresponding mono-addition product with slightly shorter period (110 min) to achieve a yield around 98% (Fig. 6) than n-butyl acrylate (reaction time 125 min) under identical reaction conditions, which indicated that methyl acrylate is suitable to activate the piperidine for aza-Michael addition reaction. In a comparison of six-member cyclic amine (piperidine) and five-member cyclic amine (pyrrolidine), pyrrolidine was suitable to activate methyl acrylate (Table 1). Moreover, aliphatic amine, such as dibutylamine with methyl acrylate gave the corresponding addition product in high yield with a longer period (150 min) than the other two cyclic amines as shown in Table 1.
Subsequently, the chemoselectivity of CuBTC was examined using a mixture of 2 mmol of piperidine, 2 mmol of aniline, and 2 mmol of n-butyl acrylate as shown in Fig. 7. After 125 min, the aza-Michael addition product of piperidine was formed in high yield (98%) and no product was obtained with aniline under the same reaction conditions. On the other hand, the catalytic activity of CuBTC between aromatic anime, such as aniline with n-butyl acrylate as a Michael acceptor showed that aniline afforded only 62% yield of addition product after 24 h (Fig. 8). We then carried out the aza-Michael reaction using substituted aniline such as 4-chloroaniline and 4-methylaniline with n-butyl acrylate under the same reaction conditions in order to establish the good activity of CuBTC as catalyst. The catalytic activity was found to be influenced by the type of substituted aniline, and the product yield was increased in the following order: 4-chloroaniline < aniline < 4-methylaniline (Table 1). Similar observations were reported earlier for other catalysts [38, 42].
The heterogeneity of the catalyst was carried out through the catalyst recycling test (Table 1). The material was separated after each reaction by simple filtration, washed with methanol, and dried under vacuum for 5 h at 100 ℃. The recovered material was reused several times without losing any significant activity (Table 1). No Cu leaching in the filtrate mixture was found by the atomic absorption spectrometer. XRD and SEM results of reused catalyst suggesting that the stability of CuBTC was retained during the reaction (Figs. 1 and 2). The IR spectrum of the reused catalyst was also indistinguishable to the fresh CuBTC (Fig. 4b).
To further examine the leaching of active metal in homogeneous phase, the aza-Michael addition reaction of piperidine with n-butyl acrylate was performed at room temperature using CuBTC as a catalyst and the catalyst was filtered off after 60 min of reaction time. The filtrate mixture (without catalyst) was continued for reaction with stirring at room for further 65 min as shown in Fig. 9. No significant increase activity was found after the separation of catalyst, indicating the reaction proceed through Cu sites in the MOF matrix.
A proposed reaction mechanism for the aza-Michael addition reaction of amine to electron-deficient alkene over heterogeneous CuBTC catalyst is shown in Fig. 10. The mechanism involves the initial coordination of carbonyl oxygen of Michael acceptor to unsaturated Cu(II) open metal sites to rise the electrophilicity of the β-carbon (I) [38]. This intermediate species reacts with the amine to generate a new carbon–nitrogen bond (II), and simultaneously form (III) by intermolecular proton transfer. In the final step, the corresponding product was formed and releasing the CuBTC [38, 43].
4 Conclusion
In summary, we have described an efficient heterogeneous catalyst system for the Aza-Michael reaction for cyclic, aliphatic, and aromatic amines with electron-deficient alkenes using CuBTC under mild reaction conditions. Cyclic and aliphatic amines were better to activate alkenes over CuBTC with high product yield (⁓99%) at room temperature within 2 h reaction time. The catalyst showed high product yields and excellent chemo-selectivity between the aromatic amine and cyclic amine. The catalyst could be successfully recycled for at least four recycles without any loss of catalytic activity. CuBTC can be easily prepared and showed high stability in C–C bond formation, which makes this protocol attractive for modern chemical industries.
References
Rulev AY (2011) Aza-Michael reaction: achievements and prospects. Russ Chem Rev 80:197–218
Sánchez-Rosellό M, Aceña JL, Simόn-Fuentes A, Pozo del CA (2014) A general overview of the organocatalytic intramolecular aza-Michael reaction. Chem Soc Rev 43:7430–7453
Cabral J, Laszlo P, Mahe L, Montaufier MT, Randriamahefa SL (1989) Catalysis of the specific michael addition: The example of acrylate acceptors. Tetrahedron Lett 30:3969–3972
Genest D, Portinha D, Fleury E, Ganachaud F (2017) The aza-Michael reaction as an alternative strategy to generate advanced silicon-based (macro) molecules and materials. Prog Poly Sci 72:61–110
Xu LW, Li L, Xia CG (2004) Transition-Metal-Based Lewis Acid Catalysis of Aza-Type Michael Additions of Amines to α, β-Unsaturated Electrophiles in Water. Helv Chim Acta 87:1522–1526
Azizi N, Saidi MR (2004) LiClO4 Accelerated Michael addition of amines to α, β-unsaturated olefins under solvent-free conditions. Tetrahedron 60:383–387
Xu LW, Li JW, Xia CG, Zhou SL, Hu XX (2003) Efficient Copper-Catalyzed Chemo Selective Conjugate Addition of Aliphatic Amines to α, β-Unsaturated Compounds in Water. Synlett 15:2425–2427
Bartoli G, Bosco M, Marcantoni E, Pertrini M, Sambri L, Torregiani E (2001) Conjugate Addition of Amines to α, β-Enones Promoted by CeCl3·7H2O−NaI System Supported in Silica Gel. J Org Chem 66:9052–9055
Srivastava N, Banik BK (2003) Bismuth Nitrate-Catalyzed Versatile Michael Reaction. J Org Chem 68:2109–2114
Giovanna B, Roderick A (2016) Aza-Michael Mono-addition Using Acidic Alumina under Solventless Conditions. Molecules 21:815
Kantam ML, Neelima B, Reddy CV, Chakravarti R (2007) Aza-Michael Addition of Imidazoles to α, β-Unsaturated Compounds and Synthesis of β-Amino Alcohols via Nucleophilic Ring Opening of Epoxides Using Copper(II) Acetylacetonate (Cu(acac)2) Immobilized in Ionic Liquids. Ind Eng Chem Res 46:8614–8619
Ying A, Wang L, Deng H, Chen J, Chen X, Ye W (2009) Aza-Michael addition of aliphatic or aromatic amines to α, β-unsaturated compounds catalyzed by a DBU-derived ionic liquid under solvent-free conditions. Tetrahedron Lett 50:1653–1657
Ghasemi MH, Kowsari E, Shafiee A (2016) Aza-Michael-type addition reaction catalysed by a supported ionic liquid phase incorporating an anionic heteropoly acid. Tetrahedron Lett 57:1150–1153
Kumar S, Kaur A, Singh V (2019) Efficient protocol for Aza-Michael addition of N-heterocycles to α, β-unsaturated compound using [Ch]OH and [n-butyl urotropinium] OH as basic ionic liquids in aqueous/solvent free conditions. Synth Commun 49:193–201
Boruah K, Borah R (2019) Design of water stable 1,3-Dialkyl- 2-methyl imidazolium basic ionic liquids as reusable homogeneous catalysts for aza-michael reaction in neat condition. ChemistrySelect 4:3479–3485
Kantam ML, Neelima B, Reddy CV (2005) A recyclable protocol for aza-Michael addition of amines to α, β-unsaturated compounds using Cu-Al hydrotalcite. J Mol Catal A: Chem 241:147–150
Esteves PA, Esloa M, Rodriguez LM, Olivia-compost A, Radim H (2007) Aza-Michael reactions with vinyl sulfones and Amberlyst-15 as catalyst. Tetrahedron Lett 48:9040–9043
Nath J, Chaudhuri MK (2009) Phosphate Impregnated Titania: An Efficient Reusable Heterogeneous Catalyst for Aza-Michael Reactions under Solvent-Free Condition. Catal Lett 133:388–393
Saidi MR, Pourshojaei Y, Aryanasa F (2009) Highly Efficient Michael Addition Reaction of Amines Catalyzed by Silica-Supported Aluminum Chloride. Synth Commun 39:1109–1119
Bosica G, Spiteri J, Borg C (2014) Aza-Michael reaction: selective mono- versus bis-addition under environmentally-friendly conditions. Tetrahedron 70:2449–2454
Kalita P, Pegu CD, Dutta P, Baruah PK (2014) Room temperature solvent free aza-Michael reactions over nano-cage mesoporous materialsJ. Mol Catal A 394:145–150
Hosseinzadeh R, Aghili N (2016) M. Synthesis, Characterization and Catalytic Application of MCM-41 Supported Phenanthrolinium Dibromide Catalyst for Aza Michael Addition Reaction in Aqueous Medium. Catal Lett 146:1194–1203
Rathod PB, Kumar KSA, Athawale AA, Pandey AKS, Chattopadhyay S (2018) Polymer-Shell-Encapsulated Magnetite Nanoparticles Bearing Hexamethylenetetramine for Catalysing Aza-Michael Addition Reactions. Eur J Org Chem 43:5980–5987
Hakiki A, Kerbadou RM, Boukoussa B, Zahmani HH, Launay F, Pailleret A, Pillier F, Hacini S, Bengueddach A, Hamacha RJ (2019) Inorg Organomet Poly Mater 29:1773–1784
Corma A, Garcia H, LlabrésiXamena FX (2010) Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem Rev 110:4606–4655
Bhattacharjee S, Lee YR, Puthiaraj P, Cho SM, Ahn WS (2015) Metal-Organic Frameworks for Catalysis. Catal Surv Asia 19:203–222
Hu ML, Safarifard V, Doustkhah E, Rostamnia S, Morsali A, Nouruzi N, Beheshti S, Akhbari K (2018) Taking organic reactions over metal-organic frameworks as heterogeneous catalysis. Micropor Mesopor Mater 256:111–127
Rostamnia S, Mohsenzad F (2018) Nanoarchitecturing of open metal site Cr-MOFs for oxodiperoxo molybdenum complexes [MoO(O2)2@En/MIL-100(Cr)] as promising and bifunctional catalyst for selective thioether oxidation. Mol Catal 445:12–20
Rostamnia S, Alamgholiloo H, Jafari M (2018) Ethylene diamine post-synthesis modification on open metal site Cr-MOF to access efficient bifunctional catalyst for the Hantzsch condensation reaction. Appl Organomet Chem 32:e4370
Alamgholiloo H, Zhang S, Ahadi A, Rostamnia S, Banaei R, Li Z, Liu X, Shokouhimehr M (2019) Synthesis of bimetallic 4-PySI-Pd@Cu(BDC) via open metal site Cu-MOF: Effect of metal and support of Pd@Cu-MOFs in H2 generation from formic acid. Mol Catal 467:30–37
Panahi P, Nouruzi N, Doustkhah E, Mohtasham H, Ahadi A, Ghiasi-Moaser A, Rostamnia S, Mahmoudi G, Khataeed A (2019) Zirconium based porous coordination polymer (PCP) bearing organocatalytic ligand: A promising dual catalytic center for ultrasonic heterocycle synthesis. Ultrason Sonchem 58:104653
Alamgholiloo H, Rostamnia S, Zhang K, Lee TH, Lee YS, Varma RS, Jang HW, Shokouhimehr M (2020) Boosting Aerobic Oxidation of Alcohols via Synergistic Effect between TEMPO and a Composite Fe3O4/Cu-BDC/GO Nanocatalyst. ACS Omega 5:5182–5191
Alamgholiloo H, Rostamnia S, Hassankhani A, Liu X, Eftekhari A, Hasanzadeh A, Zhang K, Karimi-Maleh H, Khaksar S, Varma RS, Shokouhimehr M (2020) Formation and stabilization of colloidal ultra-small palladium nanoparticles on diamine-modified Cr-IL-101: Synergic boost to hydrogen production from formic acid. J Colloid Interf Sci 567:126–135
Jang MS, Yu K, Lee J, Ahn WS (2020) Sonochemical synthesis of rho-ZMOF catalyst for an enhanced CO2 cycloaddition reaction. Mater Lett 277:128387
Kim J, Cho HY, Ahn WS (2012) Synthesis and Adsorption/Catalytic Properties of the Metal Organic Framework CuBTC. Catal Surv Asia 16:106–119
Hall J, Bollini P (2019) Structure, characterization, and catalytic properties of open-metal sites in metal organic frameworks. React Chem Eng 4:207–222
Kökçam-Demir U, Goldman A, Esrafili L, Gharib M, Morsali A, Weingart O, Janiak C (2020) Coordinatively Unsaturated Metal Sites (Open Metal Sites) in Metal-Organic Frameworks: Design and Applications. Chem Soc Rev 49:2751–2798
Rostamnia S, Alamgholiloo H (2018) Synthesis and Catalytic Application of Mixed Valence Iron (FeII/FeIII)-Based OMS-MIL-100(Fe) as an Efficient Green Catalyst for the azaMichael Reaction. Catal Lett 148:2918–2928
Savonnet M, Aguado S, Ravon U, Bazer-Bachi D, Lecocq V, Bats N, Pinel C, Farrusseng D (2009) Solvent free base catalysis and transesterification over basic functionalised Metal-Organic Frameworks. Green Chem 11:1729–1732
Nguyen LTL, Nguyen TT, Nguyen KD, Phan NTS (2012) Metal–organic framework MOF-199 as an efficient heterogeneous catalyst for the aza-Michael reaction. Appl Catal A Gen 425–426:44–52
Kim J, Kim SH, Yang ST, Yang ST, Ahn WS (2012) Bench-scale preparation of Cu3(BTC)2 by ethanol reflux: Synthesis optimization and adsorption/catalytic applications. Micropor Mesopor Mater 161:48–55
Douraki SM, Massah AR (2015) The zeolite ZSM-5-SO3H catalyzed aza-Michael addition of amines and sulfonamides to electron-deficient alkenes under solvent-free conditions. Indian J Chem 54B:1346–1349
Dai L, Zhang Y, Dou Q, Wang X, Chen Y (2013) Chemo/regioselective Aza-Michael additions of amines to conjugate alkenes catalyzed by polystyrene-supported AlCl3. Tetrahedron 69:1712–1716
Acknowledgements
This work was supported by University of Dhaka (CARS) internal fund and in part by the Basic Science Research Program through the National Research Foundation of Korea (NRF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhattacharjee, S., Shaikh, A.A. & Ahn, WS. Heterogeneous Aza-Michael Addition Reaction by the Copper-Based Metal–Organic Framework (CuBTC). Catal Lett 151, 2011–2018 (2021). https://doi.org/10.1007/s10562-020-03459-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03459-7