Abstract
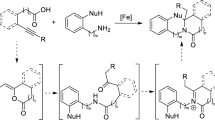
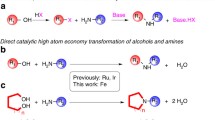
The synthesis of imines by oxidative self- or cross-condensation of primary amines has been achieved using iron(II) bromide as the catalyst. The protocol is ligand-free, additive-free, solvent-free, and high-yielding and utilizes renewable oxygen as sole oxidant. The reaction tolerated a wide range of functionalities.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Oxidative condensation reactions are important transformations in organic synthesis, which are largely promoted by metals such as Pd, Ru, Cu, and Ni catalysts [1, 2]. Recently, we reported analogous C–C, C–N, C–O, and C–S bond-forming processes using iron compounds as the catalysts [3, 4]. In general, iron compounds are of great synthetic interest because they possess benign character and abundant [5–11].
Imines are versatile intermediates for the synthesis of pharmaceutically and biologically active compounds, and fine chemicals [12–14]. The C=N bond in imines is also widely used in organic transformations such as addition, reduction, cyclization, and aziridination reactions [15–20]. Imines have traditionally been synthesized through condensation of primary amines with aldehydes or ketones, but alternative mild and practical catalytic methods [21–24], including oxidative coupling of alcohols and amines [25–30] together with oxidation of secondary amines [31–33] have also been developed. For a long time, little attention had been given to the oxidation of primary amines probably due to poor product selectivity [34]. The discovery of oxidative catalytic systems that operate effectively with molecular oxygen or air as the sole oxidant has contributed to revitalize interest in this area [35]. Recently, non-precious transition-metal catalysis [36–42], metal-free catalysis [43–51], bioinspired catalysis [47–51], and photocatalysis [52–59] have led to homo-coupled imines in high yields and selectivity. However, these coupling reactions suffer from two major draw backs that limits the synthetic applications of imines. Firstly, most of these reactions are performed in solvent media. Solvents are perhaps the most crucial parameters in industrial chemical synthesis; they represent an important challenge because solvents often account for the vast majority of mass waste in the processes [60]. Secondly, the condensation reactions of amines are not efficient methods to prepare cross-coupled imines due to the intrinsic self-coupling properties of the substrates [36, 43, 61, 62]. To the best of our knowledge, only a few efficient and selective (>95 %) oxidative cross-coupling protocols for diverse amines have been reported to date [48, 63–65].
In the course of our research on the development of new methods for nitrogen heterocycles syntheses [3, 4], we found that N-benzylidenebenzylamines can readily be obtained from the iron-catalyzed oxidative self-condensation (homo-coupling) of benzylamines. We then, intended to explore a generalized method for the preparation of various functionalized imines. Herein, we report an efficient solvent-free process for the synthesis of imines by oxidative self and/or cross-condensation of primary amines using iron(II) bromide as catalyst and molecular oxygen as sole oxidant.
2 Results and Discussion
The oxidative self-condensation of benzylamine was chosen as a model reaction to optimize the reaction conditions (Table 1). Taking into account the observations made in the oxidative condensation of benzylamines with o-phenylenediamines [3], a reagent system of iron(II) bromide, molecular oxygen and chlorobenzene was tested (entry 1; Table 1). To our delight, amine 1a self-coupled smoothly at 110 °C to give N-benzylidenebenzylamine (2a) in 90 % yield. Comparing with many other transition metal-catalyzed reactions [36–42], this reaction system was quite simple because no ligand or additives were required. A quick survey of different iron salts indicated that iron(II) bromide was superior to other catalysts (entries 2–10; Table 1). Strikingly, the yields of imine 2a were slightly higher in solvent-free conditions than the chlorobenzene solution by simply testing for some iron salts (entries 2, 7,10). Gratifyingly, the iron(II) bromide reaction in the absence of solvent produced the highest yield (97 %, entry 11; Table 1). The yield of imine 2a was decreased when the reaction was carried out in air atmosphere or at low temperature due to the incomplete reaction (entries 12, 13; Table 1). Investigation of the catalyst loading showed that 5 mol% of iron(II) bromide was required. The yield of 2a was reduced to 71 % when 2 mol% iron(II) bromide was used (entry 14; Table 1). In the absence of iron catalyst, the reaction proceeded very slowly, and the conversion of benzylamine to N-benzylidenebenzylamine reached 53 % in seven days (entry 15; Table 1). Furthermore, the reaction failed to give the desired imine compound when the process was carried out under a N2 atmosphere. The best catalytic activity of FeBr2 under solvent-free conditions, compared with the other iron salts, is possibly associated with the fact that the bromide is a better leaving group than chloride. This leads to a vacant coordination site on iron(II), thus allowing easy coordination of substrate to the iron(II) centre. Furthermore, the catalytic system requires a one electron change in oxidation state from iron(II) to iron(III), which is more feasible than the conversion of iron(III) to iron(IV), thus making the catalyst iron(III) bromide less useful than iron(II) bromide. Therefore, we decided to perform the subsequent reactions of the benzylamines in the presence of iron(II) bromide as catalyst (5 mol%) and molecular oxygen as oxidant at 110 °C under solvent-free conditions.
The scope of the oxidative self-condensation of benzylamines under optimized conditions is outlined in Table 2. Substituents at the ortho, meta, and para positions of the phenyl ring of the amine (entries 1–7; Table 2) are easily accommodated. The reaction is also tolerant of electronic perturbation: oxidative self-condensation of benzylamines bearing electron-donating (entries 1–3; Table 2) and electron-withdrawing substituents (entries 4–7; Table 2) proceeded in excellent yields in short times. Functional groups such as methoxy (entry 2; Table 2), acetal (entry 3; Table 2), halogens (entries 4–6; Table 2), and trifluoromethyl (entry 7; Table 2) were shown to be highly compatible with the present reaction conditions. It is noteworthy that the halogen-substituted benzylamines performed well, leading to the halogen-substituted imine compounds (entries 4–6; Table 2), which could be used for further transformations along with the imine functionality [66]. Sterically hindered substrate such as (±)-α-methylbenzylamine also underwent efficient oxidative self-condensation (entry 8; Table 2). Furthermore, the bulkier 1-naphthalenemethylamine could be used as the substrate, leading to formation of the corresponding imine product 2j in 89 % yield (entry 9; Table 2). Heterocyclic methylamines namely furfurylamine and 2-thiophenemethylamine, could also be converted into the corresponding self-coupled imines in high yields indicating a good tolerance of heteroatoms (entries 10, 11; Table 2). In addition, aliphatic amines such as n-octylamine and cyclohexylamine were quite effective in the reaction, affording the desired products 2m and 2n in 53 and 61 % yields, respectively (entries 12, 13; Table 2). Interestingly, FeBr2/O2 catalytic system could efficiently oxidize the secondary amines viz, dibenzylamine and 1,2,3,4-tetrahydroisoquinoline to the corresponding imines in high yields (92–95 %), which represents the versatility of the present reaction protocol (entries 14, 15; Table 2).
Recently, Xu and co-workers reported Fe(NO3)3/TEMPO-catalyzed aerobic oxidation of amines to imines in toluene with 5–10 mol% catalyst loading.[40] This [Fe]/TEMPO catalyst system could oxidize benzylic amines to imines efficiently, but insignificant reaction or failed to oxidize hetero aryl methylamines and aliphatic amines. Moreover, in this catalytic system the reaction efficiency was largely affected by substituents on the phenyl ring of amine, which is not observed in our method. Also, for the oxidation of ortho-substituted benzylamines, the yields for the imine products were not notable. However, a longer reaction time for all the conversions was required compared with the system reported here. Besides the above limitations, an expensive TEMPO was used as a co-catalyst, which is not recovered and reused.
Encouraged by excellent yields of the oxidative self-condensation of benzylamines, our efforts were next focused to explore the scope of cross-condensation between benzylamines 1 and primary amines 3 (Table 3). First, the reaction between various benzylamines and aniline was investigated under standard conditions (entries 1–6; Table 3). All the cross-condensation reactions were carried out with one molar equivalent of benzylamines and 1.2 molar equivalents of primary amines. To our delight, benzylamines reacted smoothly with aniline furnished the N-benzylideneanilines 4a–f in good to excellent yields. As previously reported by our group [3], it is likely that the self-condensation products N-benzylidenebenzylamines were formed first and then reacted with aniline to form N-benzylideneanilines by transimination. The yields of the reactions of benzylamines bearing electron-donating groups with aniline were slightly higher in comparison with electron-withdrawing amines.
Next, we turned our attention to study the effect of the reactions of benzylamine with various primary amines under standard conditions (entries 7–18; Table 3). In these reactions, the electronic properties of groups on the phenyl ring of anilines had some effects on the catalytic activities. Both electron-donating (e.g., Me, iPr, OMe) and electron-withdrawing (e.g., F, Cl, Br) substituents were tolerated in the reaction. Generally, anilines having an electron-donating substituent on the phenyl moiety gave slightly higher yield of cross-coupled imine products than the electron-withdrawing ones (entries 7–15; Table 3). This is likely due to the reduced basicity of the anilines containing electron-withdrawing groups which makes the transimination step sluggish. Steric effects of substituents had little effect on the yield of the reaction. For example, the reaction of 4- and 2-methylanilines with benzylamine resulted in the formation of N-benzylidene-4-methylaniline (4g) and N-benzylidene-2-methylaniline (4h) in 96 and 91 % yields, respectively (entries 7, 8; Table 3). Furthermore, aliphatic amines such as n-octylamine, n-butylamine and cyclohexylamine were performed well in the cross-condensation reaction, furnished the corresponding imines 4p–r in 78, 69 and 75 % yields, respectively (entries 16–18; Table 3).
To examine the feasibility of a large-scale experiment, the reaction of 4-fluorobenzylamine with aniline was investigated. The reaction could afford 1.81 g of imine 4d in 91 % yield without any significant loss of its efficiency (Scheme 1). Therefore, this protocol could be used as a practical method to synthesize the precursors of some important biologically active molecules, such as triaryl-1,2,4-oxadiazole 5, which shows significant inhibitory cytotoxicity against MCF7 and K562 cell lines [67].
To explore the reaction mechanism, the following experiments were performed as shown in Scheme 2. Benzylamine treated with iron(II) bromide and molecular oxygen to provide homo-coupled imine 2a in 97 % yield (Scheme 2A). Whereas, only a trace amount of 2a was observed in the absence of Fe source (Scheme 2B). This experiment demonstrates the essential role of the iron catalyst for this reaction. Next, the reaction of imine 2a with aniline in the presence of iron catalyst afforded the cross-coupled imine 4a in 98 % yield in 3 h (Scheme 3A). Unfortunately, the transimination reaction to form imine 4a did not occur in the absence of catalyst in 3 h (Scheme 3B). However, after a prolonged reaction time (40 h), investigating the reaction mixture showed that the imines 2a and 4a are present in 3:1 ratio by 1H NMR analysis (Scheme 3B). Similarly, the transimination of 2a with n-octylamine in the presence of FeBr2 led to the corresponding cross-coupled imine in 57 % yield in 12 h, whereas no cross-imine compound was detected in the absence of catalyst at the same interval. Thus, the iron catalyst would be essential to promote both the self- and cross-condensation reactions of amines. We presumed that the cross-condensation reaction take place through the homo-coupled imine.
According to the above observations, a tentative mechanism for the formation of homo and cross-coupled imines was proposed based on previous reports in the literature [3, 63–65]. As shown in Scheme 4, the first step may involve the oxidative addition of iron to the amine forming the complex 6. Further oxidation of 6, provides benzylimine (7) and regenerate the iron(II) bromide for catalytic cycle. Reaction of imine 7 with another benzylamine leads to the homo-coupled product 2a with liberation of ammonia. Next, the nucleophilic addition of aniline to imine 2a takes place in the presence of iron catalyst to give iron-diamine complex 8. Finally, diamine complex 8 breaks down to the cross-imine product 4a and iron-amine complex 6 (regenerated).
3 Conclusions
In summary, a practical and convenient synthetic method in solvent-free conditions using iron(II) bromide as the catalyst (5 mol%) and molecular oxygen as the oxidant has been developed for the facile synthesis of functionalized homo- and cross-coupled imines from primary amines. The operational simplicity, broad substrate scope, and excellent yields of the products are the main advantages of this method. The tolerance of diverse functional groups makes the present protocol more attractive. Furthermore, this procedure is cheap, safe and environmentally benign.
References
Li BJ, Shi ZJ (2012) Chem Soc Rev 41:5588
Shi W, Liu C, Lei A (2011) Chem Soc Rev 40:2761
Gopalaiah K, Chandrudu SN (2015) RSC Adv 5:5015
Gopalaiah K, Chandrudu SN, Devi A (2015) Synthesis 47:1766
Bauer I, Knolker HJ (2015) Chem Rev 115:3170
Gopalaiah K (2013) Chem Rev 113:3248
Bezier D, Sortais JB, Darcel C (2013) Adv Synth Catal 355:19
Sun CL, Li BJ, Shi ZJ (2011) Chem Rev 111:1293
Bauer EB (2008) Curr Org Chem 12:1341
Plietker B (2008) Iron catalysis in organic chemistry. Wiley, Weinheim
Bolm C, Legros J, Paih JL, Zani L (2004) Chem Rev 104:6217
Murahashi SI (1995) Angew Chem Int Ed 34:2443
Yao S, Saaby S, Hazell RG, Jørgensen KA (2000) Chem - Eur J 6:2435
Liu Z-Y, Wang Y-M, Li Z-R, Jiang J-D, Boykin DW (2009) Bioorg Med Chem Lett 19:5661
Kobayashi S, Ishitani H (1999) Chem Rev 99:1069
Adams JP (2000) J Chem Soc Perkin Trans 1(2):125
Chen D, Wang Y, Klankermayer J (2010) Angew Chem Int Ed 49:9475
Kobayashi S, Mori Y, Fossey JS, Salter MM (2011) Chem Rev 111:2626
He R, Jin X, Chen H, Huang Z-T, Zheng Q-Y, Wang C (2014) J Am Chem Soc 136:6558
Kondo M, Kobayashi N, Hatanaka T, Funahashi Y, Nakamura S (2015) Chem Eur J 21:9066
Patil RD, Adimurthy S (2013) Asian J Org Chem 2:726
Angelici RJ (2013) Catal Sci Technol 3:279
Qin W, Long S, Panunzio M, Biondi S (2013) Molecules 18:12264
Ryland BL, Stahl SS (2014) Angew Chem Int Ed 53:8824
Chen B, Wang L, Gao S (2015) ACS Catal 5:5851
Soulé JF, Miyamura H, Kobayashi S (2013) Chem Commun 49:355
Chen B, Li J, Dai W, Wang L, Gao S (2014) Green Chem 16:3328
Han L, Xing P, Jiang B (2014) Org Lett 16:3428
Guan M, Wang C, Zhang J, Zhao Y (2014) RSC Adv 4:48777
Tamura M, Tomishige K (2015) Angew Chem Int Ed 54:864
Zhu B, Angelici RJ (2007) Chem Commun 21:2157
Wendlandt AE, Stahl SS (2014) J Am Chem Soc 136:506
Wendlandt AE, Stahl SS (2014) J Am Chem Soc 136:11910
Schümperli MT, Hammond C, Hermans I (2012) ACS Catal 2:1108
Largeron M (2013) Eur J Org Chem 24:5225
Patil RD, Adimurthy S (2011) Adv Synth Catal 353:1695
Hu Z, Kerton FM (2012) Org Biomol Chem 10:1618
Patil RD, Adimurthy S (2012) RSC Adv 2:5119
Huang B, Tian H, Lin S, Xie M, Yu X, Xu Q (2013) Tetrahedron Lett 54:2861
Zhang E, Tian H, Xu S, Yu S, Xu Q (2013) Org Lett 15:2704
Wang J, Lu S, Cao X, Gu H (2014) Chem Commun 50:5637
Zhao S, Liu C, Guo Y, Xiao J-C, Chen Q-Y (2014) J Org Chem 79:8926
Huang H, Huang J, Liu Y-M, He H-Y, Cao Y, Fan KN (2012) Green Chem 14:930
Su C, Acik M, Takai K, Lu J, Hao S-J, Zheng Y, Wu P, Bao Q, Enoki T, Chabal Y-J, Loh KP (2012) Nat Commun 3:1298
Liu L, Wang Z, Fu X, Yan C-H (2012) Org Lett 14:5692
Wang H, Zheng X, Chen H, Yan K, Zhu Z, Yang S (2014) Chem Commun 50:7517
Largeron M, Chiaroni A, Fleury M-B (2008) Chem - Eur J 14:996
Wendlandt AE, Stahl SS (2012) Org Lett 14:2850
Yuan H, Yoo W-J, Miyamura H, Kobayashi S (2012) J Am Chem Soc 134:13970
Largeron M, Fleury M-B (2012) Angew Chem Int Ed 51:5409
Largeron M, Fleury M-B (2013) Science 339:43
Lang X, Chen X, Zhao J (2014) Chem Soc Rev 43:473
Naya SI, Kimura K, Tada H (2013) ACS Catal 3:10
Li N, Lang X, Ma W, Ji H, Chen C, Zhao J (2013) Chem Commun 49:5034
Zhao W, Liu C, Cao L, Yin X, Xu H, Zhang B (2013) RSC Adv 3:22944
Yang X-J, Chen B, Li X-B, Zheng L-Q, Wu L-Z, Tung C-H (2014) Chem Commun 50:6664
Ye L, Li Z (2014) ChemCatChem 6:2540
Yuan B, Chong R, Zhang B, Liu J, Li C (2014) Chem Commun 50:15593
Sun D, Ye L, Li Z (2015) Appl Catal B 164:428
Constable DJC, Curzonsb AD, Cunningham VL (2002) Green Chem 4:521
Tayade KN, Mishra M (2014) J Mol Catal A 382:114
Mo-nopoli A, Cotugno P, Iannone Iannone, Ciminale F, Dell’Anna MM, Mastrorilli P, Nacci A (2014) Eur J Org Chem 27:5925
Qiu X, Len C, Luque R, Li Y (2014) ChemSusChem 7:1684
Largeron M, Fleury M-B (2015) Chem Eur J 21:3815
Murray AT, King R, Donnelly JVG, Dowley MJH, Tuna F, Sells D, John MP, Carbery DR (2016) ChemCatChem 8:510
Brown HC, Okamoto Y (1958) J Am Chem Soc 80:4979
Miralinaghi P, Salimi M, Amirhamzeh A, Norouzi M, Kandelousi HM, Shafiee A, Amini M (2013) Med Chem Res 22:4253
Acknowledgments
This work was supported by the University of Delhi through R & D Grant and DU/DST-Purse Grant for promoting the Basic Research in the University. A.S. give thanks the University Grants Commission for fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Pavan Mathur on the occasion of his 65th birthday.
Rights and permissions
About this article
Cite this article
Gopalaiah, K., Saini, A. A Solvent-Free Process for Synthesis of Imines by Iron-Catalyzed Oxidative Self- or Cross-Condensation of Primary Amines Using Molecular Oxygen as Sole Oxidant. Catal Lett 146, 1648–1654 (2016). https://doi.org/10.1007/s10562-016-1789-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1789-3