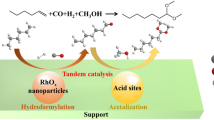
Abstract
The acetal formation mechanism under acid-free Rh-catalyzed hydroformylation–acetalization condition has been studied using different rhodium catalyst precursors in MeOH. In the absence of added acidic co-catalyst, the acetalization is catalyzed by the H+ formed in situ under hydroformylation condition, and Rh active site on Rh-phosphine catalyst did not exhibit catalytic activity for acetalization. Whether H+ can be generated in situ is related with the structure of rhodium catalyst precursor. Under hydroformylation condition, added Brønsted acids as co-catalysts can improve acetalization efficiency, but the H+ concentration in the system should not be excessively high to avoid the acid-induced inhibition for hydroformylation.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Rhodium-catalyzed hydroformylation of alkenes is an important method for production of aldehydes in industry. Based on reactivity of aldehyde group [1] and low energy consumption principle in green chemistry, the hydroformylation can be integrated with numerous other organic reactions to form the tandem reactions, such as tandem hydroformylation–reduction, hydroformylation–nucleophilic addition and hydroformylation–aldol condensation, etc. [2]. Thereinto, the tandem hydroformylation–acetalization is a typical hydroformylation–nucleophilic addition reaction, in which the alkenes can be transformed into the acetal via a one-pot synthesis procedure. Acetal formation is an important reaction in organic synthesis and frequently used to protect the aldehyde group or to further synthesize other valuable chemicals like perfume, pharmaceuticals and agricultural chemicals.
To generate acetals, the hydroformylation of olefins is generally carried out in alcohols or orthoesters in the presence of Lewis acids [3–5] or Brønsted acids [6–10] as co-catalysts. Although some Rh-catalyzed tandem hydroformylation–acetalization reactions under non-acidic conditions has been reported recently [11–14], most of these studies placed emphasis on catalysts, reaction kinetics and reaction scope, and the mechanism of acetal formation in the absence of acidic co-catalysts has received little attention.
The purpose of present study is to investigate the mechanism of acetal formation in acid-free Rh-catalyzed hydroformylation–acetalization system and the effects of different Rh precursors, acid co-catalysts and ligands structures on acetal formation.
2 Experimental
2.1 General Procedures
1-Octene was purchased from Acros company. Rh(acac)(CO)2 and RhCl3·3H2O were purchased from ABCR company. RhCl(CO)(PPh3)2 was purchased from Strem Chemicals, Inc. [Rh(COD)Cl]2 and [Rh(COD)2]BF4 were synthesized according to literature report [15]. The 4-(diphenylphosphino)-dl-phenylglycine (1) was synthesized by a published method [16]. Other reagents were obtained from commercial sources. The hydroformylation–acetalization was carried out in a homemade stainless steel autoclave with magnetic stirring under an argon atmosphere using standard Schlenk techniques. The solvents and reagents were rigorously deoxygenated prior to use. The n-nonanal was distilled to remove the n-nonoic acid formed from oxidation of n-nonanal prior to use. The conversion and selectivity were determined by GC with an OV-101 capillary column. The products have been identified by GC–MS (Agilent 6890/5973 GC–MS apparatus with a DB-35MS capillary column).
2.2 General Procedure for Hydroformylation–Acetalization of 1-Octene Using Different Rhodium Catalyst Precursors in MeOH
Rhodium catalyst precursors (3.87 × 10−3 mmol), phosphine ligands (3.87 × 10−2 mmol), 1-octene (0.6 mL, 3.82 mmol), internal standard (cyclohexane, 0.1 mL) and MeOH were transferred into a stainless steel autoclave. Then the reactor was pressurized with syngas (H2/CO) to 5.0 MPa, and the reaction system was heated to 80 °C. After 2–10 h the autoclave was rapidly cooled with ice, and the conversion and selectivity were analysed by GC.
2.3 General Procedure for Acetalization of n-Nonanal in MeOH
Rhodium catalyst precursors (3.87 × 10−3 mmol), phosphine ligands (3.87 × 10−2 mmol), n-nonanal (3.87 mmol) and MeOH were transferred into a stainless steel autoclave. Then the reactor was pressurized with syngas (H2/CO) to 5.0 MPa, and the reaction system was heated to 80 °C. After 2 h the autoclave was rapidly cooled with ice and the conversion was analysed by GC.
3 Results and Discussion
Recently, several acid-free Rh-catalyzed tandem hydroformylation–acetalization reactions have been reported [11–14], and some transition metal complexes have successfully been used for catalyzing the acetalization reactions [17], however, these reported systems cannot rule out the possibility of Brønsted acid catalysis, since H+ formed in situ may exist according to hydroformylation mechanism. We are interested in whether Rh-phosphine complexes can also catalyze the transformation of aldehyde into acetal under Rh-catalyzed hydroformylation condition without acid co-catalysts.
Although the effects of different Rh precursors on the selectivity of acetals in tandem hydroformylation–acetalization have been studed [11, 12], the mechanism of acetal formation is still not fully clear. Therefore, it is necessary to further determine the mechanisms of acetal formation in the presence of different Rh precursors.
To begin with, we evaluated the hydroformylation and acetalization efficiency in the one-pot hydroformylation–acetalization of 1-octene using different Rh precursors with triphenylphosphine (PPh3) as the model ligand (Table 1). The conversion of 1-octene and Soxo indicate the hydroformylation efficiency, while Eace indicates the acetalization efficiency. The homogeneous hydroformylation reaction was performed at 80 °C under 5.0 MPa 1:1 CO/H2 in methanol.

It can be seen from Table 1 that the excellent hydroformylation efficiency with high conversion of 1-octene and selectivity for oxo products was obtained using [Rh(COD)2]BF4 and [Rh(COD)Cl]2 precursors (Table 1, entries 1 and 2), and the acetalization efficiency reaches 32 % when using the ionic Rh precursor [Rh(COD)2]BF4 and 87 % when using the non-ionic Rh precursor [Rh(COD)Cl]2, indicating that the acetalization reaction is slightly slower than hydroformylation. Interestingly, for RhCl3·3H2O, despite the high acetalization efficiency (87 %), the conversion of 1-octene is only 12 % within 2 h (Table 1, entry 3) and 1-octene converted nearly complete only until 4 h (Table 1, entry 4). This observation suggests that when using RhCl3·3H2O as the Rh precursor, the formation of the catalytically active species is remarkably inhibited. Note that using the chlorine-free Rh(acac)(CO)2 as the precursor (Table 1, entry 5) resulted in high aldehyde selectivity with almost no formation of acetal (about 1 %). Even after prolonged reaction time of 10 h, the acetalization efficiency reached only 57 % (Table 1, entry 6), indicating that acetalization is much slower than hydroformylation when using Rh(acac)(CO)2 as the precursor.
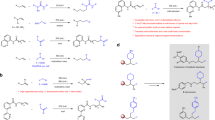
According to the hydroformylation mechanism and through analysing the above experimental results, we believe that the acetalization under hydroformylation condition is catalyzed by the Brønsted acid that formed in situ during the formation of catalytically active species. For [Rh(COD)2]BF4 and [Rh(COD)Cl]2 precursors, the formation of catalytically active species is accompanied by the generation of equimolar HBF4 (Scheme 1, (1)) [14] and HCl (Scheme 1, (2)), whereas using RhCl3·3H2O gives a three-fold amount of HCl (Scheme 1, (3) and (4)). According to the Wilkinson mechanism [19], in the reversible reaction that forms catalytically active species and H+, higher H+ concentration will inhibit the formation of catalytically active species, which explains the reduced hydroformylation efficiency (Table 1, entry 3) when using RhCl3·3H2O as the catalyst precursor. Besides, the formation of catalytically active species from the Rh(acac)(CO)2 precursor does not liberate H+, which thus results in the low acetal yield.
To confirm the acid catalysis mechanism in acetal formation, we designed two sets of experiments to evaluate the effect of acidic and basic additives on the acetalization efficiency (Table 1, entries 7–12). As expected, basically no acetal was formed in the hydroformylation systems using [Rh(COD)2]BF4, [Rh(COD)Cl]2 or RhCl3·3H2O after the addition of the strongly basic tetrabutylammonium hydroxide (TAH, 25 % solution in MeOH), since the TAH neutralized the in situ formed H+. In contrast, after the addition of equimolar HBF4 or HCl in the catalytic system using Rh(acac)(CO)2, the acetalization efficiency improved to 51 and 76 % respectively, which are close to the acetalization efficiency of using [Rh(COD)2]BF4 and [Rh(COD)Cl]2 (compare entries 1 and 10, 2 and 11 in Table 1). When further increasing the HCl concentration to three times the amount of Rh(acac)(CO)2, an inhibition similar to that observed when using RhCl3·3H2O was noted again and the conversion rate of 1-octene reached only 17 % (compare entries 3 and 12).
In the following experiments, we evaluated the effects of different ligands structures on acetal formation. The zwitterionic phosphine ligand 1 bearing an amino acid tag was selected as the model ligand (Scheme 2) [16]. Because of same native structure, when using Rh(acac)(CO)2 as the precursor, the catalytic activity and selectivity of Rh-1 are similar to those of PPh3 and the acetalization efficiency is less than 1 % (compare entries 5 and 13 in Table 1). However, when using RhCl3·3H2O as the precursor, no acid inhibition was observed and Rh-1 showed higher conversion than Rh-PPh3 (compare entries 3 and 14), while the acetalization efficiency of Rh-1 decreased and reached only 18 %. This result shows that the carboxylate group on the side chain of ligand 1 formed carboxylic acid with the in situ generated HCl, which reduced the H+ concentration in the system, thus improving hydroformylation efficiency but decreasing acetal yield.
In all cases, since the reaction is carried out in homogeneous sestem, the selectivity for total oxo products (aldehyde + acetal) is always at a high level within a range of 86–99 %. Moreover, as the acetalization efficiency improves, the selectivity for the n-acetal decreases, indicating that the n-aldehyde is more prone to acetalization than the i-aldehyde.
Although the above experimental results prove that the H+ generated during the formation of the catalytically active species in the hydroformylation reaction is the acetalization catalyst, Rh-species with Lewis acidity (may be those containing chlorine) could also contribute in the aldehyde acetalization, together with H+ which can be generated from the Rh precursors. Therefore, using the acetalization of n-nonanal in MeOH as the model reaction, we studied the effect of Rh-phosphine complexes on the acetalization efficiency under hydroformylation condition (Table 2).

In the absence of ligand and Rh, only 0.5 % of n-nonanal was converted to acetal within 2 h (Table 2, entry 1). By contrast, when using Rh(acac)(CO)2 with PPh3 (Table 2, entry 2), no obvious changes for acetalization efficiency were observed. As expected, using RhCl3·3H2O precursor (Table 2, entry 3) resulted in high acetalization efficiency (87 %) due to H+ formed in situ from RhCl3·3H2O (Scheme 1, (3) and (4)), and the chlorine-containing Rh-phosphine complexe RhCl(CO)(PPh3)2 (Scheme 1, (3) and (4)) that is the precursor of catalytically active Rh specie gave a moderate acetalization efficiency (41 %) (Table 2, entry 4). However, the existence of a catalytic mechanism involving chlorine-containing Rh-specie with Lewis acidity for acetalization still cannot be unambiguously excluded. In order to further illustrate this problem, the syngas H2/CO was replaced with Ar to avoid the formation of H+, after which RhCl(CO)(PPh3)2 did not make any contribution to the formation of acetal (Table 2, entry 5). The above results demonstrate that H+ formed in situ from the Rh precursors is the only catalyst for acetalization under the hydroformylation condition adopted in this work.
4 Conclusions
In conclusion, the mechanism of acetal formation in Rh-catalyzed tandem hydroformylation–acetalization was determined using different Rh precursors. Under non-acidic conditions, the H+ formed in situ is the catalyst of acetalization reaction. In our study, the Rh active site on the Rh-phosphine catalyst can not catalyze the transformation of aldehyde into acetal. Added Brønsted acids as co-catalysts can effectively improve acetalization efficiency, however, the acid concentration should not be too high, since excessively high H+ concentration can inhibit the formation of rhodium catalytically active species in hydroformylation.
References
Patai S (1966) The Chemistry of the Carbonyl Group. Wiley-Interscience, New York, p 1970
Eilbracht P, Bärfacker L, Buss C, Hollmann C, Kitsos-Rzychon BE, Kranemann CL, Rische T, Roggenbuck R, Schmidt A (1999) Chem Rev 99:3329
Cabrera A, Mortreux A, Petit F (1988) J Mol Catal 47:11
Parrinello G, Stille JK (1987) J Am Chem Soc 109:7122
Stille JK, Su H, Brechot P, Parrinello G, Hegedus LS (1991) Organometallics 10:1183
Balue J, Bayon JC (1999) J Mol Catal A 137:193
Diwakar MM, Deshpande RM, Chaudhari RV (2005) J Mol Catal A 232:179
Fernández E, Castillón S (1994) Tetrahedron Lett 35:2361
Soulantica K, Sirol S, Koïnis S, Pneumatikakis G, Kalck Ph (1995) J Organomet Chem 498:C10
Fernández E, Ruiz A, Claver C, Castillón S, Pólo A (1998) Chem Commun 1803
El Ali B, Tijani J, Fettouhi M (2005) J Mol Catal A 230:9
El Ali B, Tijani J, Fettouhi M (2006) Appl Catal A 303:213
Vieira CG, da Silva JG, Penna CAA, dos Santos EN, Gusevskaya EV (2010) Appl Catal A 380:125
Diebolt O, Cruzeuil C, Müller C, Vogt D (2012) Adv Synth Catal 354:670
Schenck TG, Downes JM, Milne CRC, Mackenzie PB, Boucher H (1985) Inorg Chem 24:2334
Brauer DJ, Schenk S, Roßenbach S, Tepper M, Stelzer O, Hausler T, Sheldrick WSJ (2000) Organomet Chem 598:116
Krompieca S, Penkalaa M, Szczubiałkab K, Kowalska E (2012) Coord Chem Rev 256:2057
Jin X, Zhao K, Cui F, Kong F, Liu Q (2013) Green Chem. doi:10.1039/C3GC41231H
Evans D, Osborn JA, Wilkinson G (1964) J Chem Soc 3133
Acknowledgments
We gratefully thank the financial support from National Natural Science Foundation of China (No. 20606019, 20976086), the Foundation of Key Laboratory of Oil & Gas Fine Chemicals, Ministry of Education, China (No.XJDX0908-2010-01) and the Natural Science Foundation of Qingdao, China (No. 12-1-4-3-(6)-jch).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, X., Zhao, K., Kong, F. et al. The Mechanism of Acetal Formation in Acid-Free Rh-Catalyzed Tandem Hydroformylation–Acetalization of Olefins in MeOH. Catal Lett 144, 192–196 (2014). https://doi.org/10.1007/s10562-013-1109-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-013-1109-0