Abstract
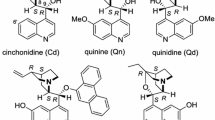
A series of polymer-bound cinchona alkaloids has been prepared. The resultant polymer-bound cinchona alkaloids have been used as the catalysts for the asymmetric Michael reaction of 1,3-dicarbonyl compounds and N-benzylmaleimide. The corresponding asymmetric Michael addition product, the first example of adjacent quaternary and tertiary stereocenters synthesized in the presence of a polymer-bound catalyst, has a selectivity of up to 86% ee. Besides, immobilized alkaloid V retains stereochemical reactivity even after being recycled for six times.
Graphical Abstract
A series of polymer-bound cinchona alkaloids has been prepared. The resultant polymer-bound cinchona alkaloids have been used as the catalysts for the asymmetric Michael reaction of 1,3-dicarbonyl compounds and N-benzylmaleimide. The corresponding asymmetric Michael addition product, the first example of adjacent quaternary and tertiary stereocenters synthesized in the presence of a polymer-bound catalyst, has a selectivity of up to 86% ee. Besides, immobilized alkaloid V retains stereochemical reactivity even after being recycled for six times.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Asymmetric Michael reaction is one of the most powerful tools for constructing stereoselective C–C bond [1–6]. However, the approach to complex compounds with quaternary stereocenters is still a challenge for synthetic organic chemists [7–9]. It has been well recognized that adjacent quaternary and tertiary stereocenters as common highly congested structural motifs in complex natural products may be constructed from simple precursors via one-step catalytic conjugate addition of compounds containing prochiral trisubstituted nucleophilic carbon atom to β-substituted Michael acceptors [10]. This, however, is infeasible unless catalysts with high levels of stereoselectivity are adopted to promote the formation of sterical C–C bond. To date, several catalytic asymmetric conjugate additions have been proven to afford 1,4-adducts containing quaternary carbon centers with excellent enantioselectivity and diastereoselectivity [11–13], and unsaturated aldehydes [14, 15], enones [16, 17] nitroalkenes [18], and unsaturated imides [19] are commonly used acceptors for the additions. The first asymmetric direct conjugate addition of 1,3-dicarbonyl compounds to maleimides, yielding highly functionalized products with adjacent quaternary and tertiary carbon centers in the presence of natural cinchona alkaloids as chiral-base catalysts, was reported by Bartoli et al. [20, 21].
Although cinchona alkaloids have long been recognized as efficient chiral catalysts for a variety of homogeneous asymmetric reactions, their utility is often limited by the difficulty of separating the product from the catalyst. Thanks to polymer-supported catalysts, the above-mentioned difficulty in separating asymmetric direct conjugate addition products from the catalysts may be overcome, since the polymer-supported catalysts can be recovered from the reaction product by simple filtration and can be reused. Several reports are currently available on polymer-supported catalysts [22, 23], but few of them deal with Michael adducts with high stereoselectivity. An encouraging exploration in that respect goes to d’Angelo and coworkers, who successfully synthesized polystyrene (Merrifield resin) supported cinchona alkaloid catalyst containing quaternary stereocenters with 87% enantioselectivity (ee) [24]. Enlightened by this and motivated by the demand to construct novel polymer-bound systems with quaternary and tertiary stereocenters, we have prepared polymer-immobilized cinchona alkaloids in the present research. This paper reports the synthesis of polymer-immobilized cinchona alkaloids and their application in asymmetric Michael reaction.
2 Experimental
2.1 General
All the reagents and solvents were purchased from commercial suppliers and used without further purification. Analytical thin layer chromatography (TLC) was performed on GF254 silica gel plates. Elemental analysis was performed on an Elementar Vario PE2400-II analyzer. The 1H and 13C NMR spectra were recorded at 400 and 100 MHz, respectively. The chemical shifts (δ) are given in parts per million (ppm) referenced to TMS. Melting points were measured with an X-6 melting point apparatus. Analytical high performance liquid chromatography (HPLC) was performed on Agilent 1100 equipped with a diode array ultraviolet (UV) detector, with which Daicel Chiralpak AD and AS columns were used.
2.2 Preparation of the Catalysts
Preparation of catalyst (I)—To quinine (2 mmol) in DMF (20 mL) was added NaH (1 mmol) at room temperature. The mixture was stirred for 1 h at 60 °C, and the Merrifield resin (0.6 g, 100–200 mesh, 1.23 mmol of Cl/g) was added to the mixture at room temperature. The mixture was stirred for an additional 3 h at 60 °C. The resin was washed with water, THF, and ether and dried under reduced pressure to afford catalyst I (0.61 mmol/g).
Preparation of catalysts (II) and (III)—Quinine (2 mmol) was dissolved in THF (6 mL/mmol). Et3N (4 mmol) and TsCl (3.6 mmol) were added parallel. The reaction system was stirred at room temperature for 2–4 h. Sat. NaHCO3 was added and the aqueous layer was extracted with CH2Cl2. The combined organic layer was dried (Na2SO4) and the solvent was removed in vacuum. The crude product was purified using column chromatograph to furnish QN-OTs. Into a round-bottomed flask filled with 20 mL triethylenetetramine was added 2.0 g Merrifield resin. After being stirred at 80 °C for 2 h, the reaction mixture was cooled to room temperature, filtered and fully washed with H2O, followed by drying in vacuum for 12 h allowing generation of brown Merrifield-triethylenetetramine (abbreviated as PS-TETA). Merrifield-phenylenediamine (PS-PPD) can be obtained in the same manner. Catalysts II (0.57 mmol/g) and III (0.51 mmol/g) were synthesized by linking the triethylenetetramine (TETA) and p-phenylenediamine spacers to the Merrifield resin at 80 °C in DMF, respectively.
Preparation of catalyst (IV) [25, 26]—A 3-necked flask (50 mL) was charged with chloromethylated styrene–divinylbenzene beads (0.3 g, 100–200 mesh, 1.23 mmol of Cl/g) and chloroform (15 mL), followed by full swelling within 2–3 h and introduction of quinine (0.3 g, 1 mmol). The mixture was heated under reflux for 50 h and then cooled. The polymer was collected by filtration and successively thoroughly washed with chloroform, methylene dichloride, and ether, then dried. The product (0.5 g), catalyst (IV), contains 0.69 mmol per gram of salt residues (determined by elemental analysis).
Preparation of catalyst (V)—3.0 g of mercaptomethyl-polystyrene (2.0 mmol/g) resin was suspended in 100 mL chloroform. Into the suspension 2.0 g of quinine and 200 mg of AIBN as radical initiator were added, followed by refluxing under a nitrogen atmosphere for 12 h. After sedimentation and removal of the solvent, the modified resin was washed with chloroform and methanol. The loading value was determined by analysis of sulfur (0.92 mmol/g for V).
Preparation of catalyst (VI)—A solution of quinine (2 mmol), methyl methacrylate (8 mmol), and AIBN (0.04 mmol) in chloroform (5 mL) was stirred magnetically for 44 h under refluxing. The precipitated polymer was filtered, washed with methanol, reprecipitated from DMF into methanol, and dried at 70 °C under vacuum. The loading value was determined by analysis of nitrogen (1.49 mmol/g for VI).
2.3 General Procedures For Catalytic Enantioselective Michael Reaction
To a solution of 1,3-dicarbonyl compound (0.2 mmol) in toluene (1.0 mL) was added N-benzylmaleimide (0.24 mmol) and the chiral catalyst (10 mol%) under stirring at room temperature. The mixture was continuously stirred until TLC analysis confirmed disappearance of the 1,3-dicarbonyl compound. The reaction mixture was then filtrated to remove the catalyst. The crude product was obtained after the solvent was evaporated and then it was purified using flash column chromatograph.
Ethyl 1-(1-benzyl-2,5-dioxopyrrolidin-3-yl)-2-oxocyclopentanecarboxylate, 3: A colourless foam. [HPLC analysis on a Daicel Chiralpak AD-H column: hexane/i-PrOH = 85/15, flow rate 0.75 mL/min, λ = 214 nm; major diastereomer: τmajor = 17.5 min, τminor = 20.5 min; minor diastereomer: τmajor = 21.8 min, τminor = 24.3 min]. 1H NMR(CDCl3): δ = 1.22 (t, J = 7.2, 3H), 1.93–2.03 (m, 2H), 2.08–2.20 (m, 1H), 2.35–2.50 (m, 3H), 2.60 (dd, J = 6.0, 18.0, 1H), 2.80 (dd, J = 9.2, 18.0, 1H), 3.46 (dd, J = 6.0, 9.2, 1H), 4.17 (q, J = 7.2, 2H), 4.53 4.59 (AB, J = 14.2, 2H), 7.18–7.35 (m, 5H); 13C NMR (CDCl3): δ = 13.9 (CH3), 19.1 (CH2), 31.6 (CH2), 32.6 (CH2), 37.9 (CH2), 42.1 (CH), 42.2 (CH2), 60.6 (C), 62.0 (CH2), 127.7 (CH), 128.3 (CH, 2C), 128.5 (CH, 2C), 135.4 (C), 169.5 (C), 175.0 (C), 177.0 (C), 213.5 (C); IR: ν = 3641, 3462, 3030, 2979, 1749, 1704, 1601,1585, 1496, 1455, 1431, 1400, 1345, 1314, 1228, 1169, 1112, 1021, 919, 830, 696, 633, 477 cm−1; HRMS: m/z calcd for C19H21NO5: 343.14197; found: 343.14173; elemental analysis calcd (%) for C19H21NO5: C 66.47, H 6.12, N 4.08; found: C 66.55, H 6.14, N 4.01.
Methyl 1-(1-benzyl-2,5-dioxopyrrolidin-3-yl)-2-oxo-2,3-dihydro-1H-indene-1-carboxylate, 4a: A colourless solid. [HPLC analysis on a Daicel Chiralpak AD-H column: hexane/i-PrOH = 75/25, flow rate 0.75 mL/min, λ = 214 nm; major diastereomer: τmajor = 18.5 min, τminor = 21.2 min; minor diastereomer: τmajor = 25.5 min, τminor = 27.5 min]. 1H NMR (CDCl3): δ = 2.50 (dd, J = 6.0, 18.0, 1H), 2.88 (dd, J = 9.2, 18.0, 1H), 3.55 3.61 (AB, J = 22.8, 2H), 3.70 (s, 3H), 3.94 (dd, J = 6.0, 9.2, 1H), 4.52 4.58 (AB, J = 14.0, 2H), 7.02–7.35 (m, 9H); 13C NMR (CDCl3): δ = 32.1 (CH2), 42.5 (CH2), 43.1 (CH2), 44.6 (CH), 53.4 (CH3), 64.8 (C), 124.5 (CH), 125.1 (CH), 127.9 (CH), 128.3 (CH), 128.6 (CH, 2C), 128.7 (CH, 2C), 129.3 (CH), 135.3 (C), 137.1 (C), 137.5 (C), 168.3 (C), 174.7 (C), 175.9 (C), 209.6 (C); HRMS: m/z calcd for C22H19NO5: 377.12632; found: 377.12603; elemental analysis calcd (%) for C22H19NO5: C 70.03, H 5.04, N 3.71; found: C 70.15, H 5.02, N 3.67.
3-(3-acetyl-2-oxotetrahydrofuran-3-yl)-1-benzylpyrrolidine-2,5-dione, 4b: A colourless solid. [HPLC analysis on a Daicel Chiralpak AD-H column: hexane/i-PrOH = 80/20, flow rate 0.75 mL/min, λ = 214 nm; major diastereomer: τminor = 29.2 min, τmajor = 37.7 min; minor diastereomer: τmajor = 21.8 min, τminor = 25.6 min]. 1H NMR (CDCl3): δ = 2.27 (s, 3H), 2.40–2.49 (m, 1H), 2.53 (dd, J = 6.4, 18.4, 1H), 2.68–2.75 (m, 1H), 2.81 (dd, J = 9.2, 18.4, 1H), 3.35 (dd, J = 6.4, 9.2, 1H), 4.27–4.34 (m, 2H), 4.61 4.67 (AB, J = 14.4, 2H), 7.23–7.33 (m, 3H), 7.32–7.36 (m, 2H); 13C NMR (CDCl3): δ = 25.9 (CH3), 28.7 (CH2), 31.7 (CH2), 42.1 (CH), 42.6 (CH2), 61.6 (C), 65.9 (CH2), 127.9 (CH), 128.5 (CH, 2C), 128.6 (CH, 2C), 135.3 (C), 173.6 (C), 174.4 (C), 176.1 (C), 200.7 (C); IR (KBr): ν = 3464, 3065, 3033, 2927, 1770, 1704, 1605, 1585, 1497, 1433, 1402, 1355, 1314, 1289, 1262, 1169, 1106, 1081, 1025, 975, 883, 696 cm−1; HRMS: m/z calcd for C17H17NO5: 315.11067; found: 315.11085; elemental analysis calcd (%) for C17H17NO5: C 64.76, H 5.40, N 4.44; found: C 64.65, H 5.48, N 4.42.
Ethyl 2-(1-benzyl-2,5-dioxopyrrolidin-3-yl)-2-methyl-3-oxobutanoate, 4c: A colourless foam. [HPLC analysis on a Daicel Chiralpak AS-H column: hexane/i-PrOH = 75/25, flow rate 0.75 mL/min, λ = 214 nm; major diastereomer: τminor = 23.2 min, τmajor = 30.8 min; minor diastereomer: τminor = 33.2 min, τmajor = 44.5 min]. 1H NMR (CDCl3): δ = 1.22 (t, J = 7.2, 3H), 1.50 (s, 3H), 2.24 (s, 3H), 2.44 (dd, J = 6.0, 18.4, 1H), 2.85 (dd, J = 9.2, 18.4, 1H), 3.37 (dd, J = 6.0, 9.2, 1H), 4.18 (q, J = 7.2, 2H), 4.61 4.67 (AB, J = 14.0, 2H), 7.23–7.40 (m, 5H); 13C NMR (CDCl3): δ = 13.9 (CH3), 18.9 (CH3), 26.8 (CH3), 32.4 (CH2), 42.4 (CH2), 44.9(CH), 61.2 (C), 62.2 (CH2), 127.8 (CH), 128.5 (CH, 2C), 128.7 (CH, 2C), 135.6 (C), 170.7 (C), 175.2 (C), 177.0 (C), 204.2 (C); IR: ν = 3462, 3033, 2983, 2941, 2565, 1957, 1775, 1701, 1605, 1585, 1497, 1455, 1431, 1401, 1352, 1314, 1293, 1171, 1096, 1018, 972, 931, 894, 757, 697 cm−1; HRMS: m/z calcd for C18H21NO5: 331.14197; found: 331.14163; elemental analysis calcd (%) for C18H21NO5: C 65.26, H 6.34, N 4.23; found: C 65.34, H 6.23, N 4.20.
3-(1-acetyl-2-oxocyclopentyl)-1-benzylpyrrolidine-2,5-dione, 4d: A colourless foam. [HPLC analysis on a Daicel Chiralpak AD-H column: hexane/i-PrOH = 75/25, flow rate 0.75 mL/min, λ = 214 nm; major diastereomer: τminor = 17.5 min, τmajor = 18.1 min; minor diastereomer: τminor = 16.2 min, τmajor = 16.6 min]. 1H NMR (CDCl3): δ = 1.80-2.00 (m, 3H), 2.19 (s, 3H), 2.35 (dd, J = 6.4, 18.4, 1H), 2.40–2.57 (m, 3H), 2.74 (dd, J = 9.2, 18.4, 1H), 3.55 (dd, J = 6.4, 9.2, 1H), 4.60 4.66 (AB, J = 14.0, 2H), 7.24–7.39 (m, 5H); 13C NMR (CDCl3): δ = 19.4 (CH2), 26.2 (CH3), 28.8 (CH2), 31.8 (CH2), 38.4 (CH2), 42.5 (CH2), 43.6 (CH), 68.7 (C), 128.0 (CH), 128.6 (CH, 2C), 128.7 (CH, 2C), 135.4 (C), 174.7 (C), 176.5 (C), 202.0 (C), 213.4 (C); IR: ν = 3461, 3033, 2964, 1775, 1742, 1704, 1601, 1497, 1431, 1400, 1352, 1314, 1287, 1208, 1167, 1084, 1028, 943, 699 cm−1; HRMS: m/z calcd for C18H19NO4: 313.13141; found: 313.13121; elemental analysis calcd (%) for C18H19NO4: C 69.01, H 6.07, N 4.47; found: C 68.96, H 6.11, N 4.42.
3-(2-acetyl-1-oxo-1,2,3,4-tetrahydronaphthalen-2-yl)-1-benzylpyrrolidine-2,5-dione, 4e: A colourless solid. [HPLC analysis on a Daicel Chiralpak AD-H column: hexane/i-PrOH = 75/25, flow rate 0.75 mL/min, λ = 214 nm; major diastereomer: τminor = 23.5 min, τmajor = 22.8 min; minor diastereomer: τminor = 26.8 min, τmajor = 22.0 min]. 1H NMR (CDCl3): δ = 2.19 (s,3H), 2.37–2.54 (m, 3H), 2.63 (dd, J = 6.0, 18.4, 1H), 2.98–3.03 (m, 2H), 3.30 (dd, J = 6.0, 9.2, 1H), 4.70 4.76 (AB, J = 14.4, 2H), 7.22-7.37 (m, 5H), 7.39-7.45 (m, 2H), 7.52 (t, J = 7.6, 9.2, 1H), 8.07 (d, J = 7.6, 9.2, 1H); 13C NMR (CDCl3): δ = 25.6 (CH2), 29.1 (CH3), 31.1 (CH2), 31.9 (CH2), 42.6 (CH2), 44.2 (CH), 65.5 (C), 127.4 (CH), 127.7 (CH), 128.1 (CH), 128.5 (CH, 2C), 128.6 (CH, 2C), 129.0 (CH), 132.1 (CH), 134.4 (C), 135.9 (C), 142.9 (C), 175.0 (C), 177.1 (C), 196.1 (C), 205.6 (C); IR (KBr): ν = 3065, 3032, 2935, 1774, 1708, 1599, 1496, 1455, 1431, 1403, 1356, 1299, 1232, 1171, 1160, 1078, 1045, 976, 913, 776, 700, 635 cm−1; HRMS: m/z calcd for C23H21NO4: 375.14706; found: 375.14643; elemental analysis calcd (%) for C23H21NO4: C 73.60, H 5.60, N 3.73; found: C 73.64, H 5.59, N 3.68.
2.4 Gram-Scale Experiment
In an ordinary 50 mL round-bottom flask equipped with a magnetic stirring bar, catalyst V (2.0 mmol) was dissolved in 20 mL of toluene. After addition of the 1,3-dicarbonyl compound 1a (20 mmol, 3.02 mL), the flask was closed with a rubber stopper and the mixture was stirred at 0 °C for 10 min. Then N-benzylmaleimide 2 (24 mmol, 4.58 g) was added in one portion and stirring was continued for 7 days. Then the crude reaction mixture was concentrated to 5 mL and directly charged on the chromatography column and purified on silica, using CH2Cl2/AcOEt 95/5 as the eluent.
3 Results and Discussion
The asymmetric Michael addition of carbon-centered nucleophiles to maleimides should supply a practicable route to synthetically and biologically momentous chiral α-substituted succinimides [19]. In the present research, the polymer-supported catalytic Michael addition of ethyl 2-oxocyclopentanecarboxylate (1a) to N-benzylmaleimide (2) has been initially focused on, and the results are summarized in Table 1. It is seen that natural cinchona alkaloid quinine [20] (QN) and cinchonine (CN) as potent catalysts for such a Michael addition afford 1,4-adduct with relatively good diastereoselectivity and enantioselectivity (Table 1, Entries 1 and 2). Besides, since quinine shows much better catalytic reactivity than cinchonine, it is chosen to be grafted with different polymer carriers. The anchorage of cinchona alkaloids at two sites was explored; the structures are depicted in Scheme 1. Unfortunately, catalyst I as an example of such polymer-grafted products containing alkaloid directly attached to the Merrifield resin shows poor chiral catalytic reactivity (Table 1, Entry 3) [27]. Therefore, spacer groups are inserted between the catalytic scaffold and polymer matrix to avoid reduce of the reactivity of quinine by direct graft of polymer support. To our disappointment, catalyst II, synthesized by linking the triethylenetetramine (TETA) spacer to the Merrifield resin, still shows fair chiral catalytic reactivity (Table 1, Entry 4); and catalyst III containing p-phenylenediamine as an alternate spacer has almost no chiral selectivity (Table 1, Entry 5). Thankfully, catalyst IV, polymer-supported chiral quaternary ammonium obtained from the reaction of a Merrifield resin with quinine by way of the bridgehead nitrogen [28] possesses greatly improved enantioselectivity (Table 1, Entry 6). This indicates that the presence of free hydroxyl group on QN is essential for achieving high level of selectivity. Moreover, thanks to the C-3 remote vinyl side chain taking part in the reaction of mercaptomethyl-polystyrene [29], catalyst V gives 85% ee, close to that of organic small molecule catalyst (Table 1, Entry 7). Also, catalyst VI, obtained via the copolymerization of QN with methyl methacrylate [30], has a high diastereoselectivity (dr) and ee value (Table 1, Entry 8). The drawback lies in that polymer-supported catalysts usually have poorer catalytic activity than corresponding organic small molecule catalysts and require extended time for synthesis. This, fortunately, could be significantly modified by adopting triethylamine (Et3N) to enhance the catalytic activity. As an example in this respect, catalytic amount of catalyst V (10 mol%) was allowed to smoothly react with Et3N (10 mol%). The resulting product, with a yield of 89%, possesses the same enantioselectivity as that of the organic small molecule catalyst listed in entry 7, and the reaction time has been greatly shortened from 20 days to 8 days (Table 1, Entry 9). Et3N may be accelerated the protonation of the succinimide moiety.

The above-mentioned results confirm that catalyst V is the best catalyst for the conjugate addition highlighted in this research. Thus catalyst V was selected as a model to optimize reaction conditions. Relevant results in this aspect are listed in Table 2. It is seen that reaction media play an important role in the reaction process. Namely, the reaction proceeded with high stereoselectivities in nonpolar solvents (tetrahydrofuran, CH2Cl2, and toluene) rather than polar solvents (MeOH, EtOH), due to formation of hydrogen bonds via either the catalyst or the substrates in the polar solvents (Table 2, Entries 1–10). Thus toluene, with ability to increase reactivity, was selected as a model solvent to conduct further investigation. For most catalytic systems, higher conversion could be achieved at elevated reaction temperature within a shorter time but generally accompanied by decreased enantioselectivity. Therefore, an excellent yield of 95%, along with a lowered ee value of 80%, was obtained within shortened reaction time when the reactions were carried out at 30 °C (Table 2, Entry 11); and the ee value for the reaction conducted at −30 °C was increased to 88% (Table 2, Entry 12). At the same time, a lower catalyst loading is favorable to promoting the conversion and increasing the enantioselectivities. Namely, 83% ee and 80% of yield were obtained after reaction for 10 days at a catalyst loading of 5 mol% (Table 2, Entry 13); and 78% of conversion and 80% ee were achieved after reaction for 16 days even at a catalyst loading of 2 mol% (Table 2, Entry 14).

Encouraged by the findings in entries 11–14 of Table 2, we further examined the conjugate addition of a variety of trisubstituted carbon Michael donors to N-benzylmaleimide (2) in the presence of catalyst V. Relevant results are presented in Table 3. It is seen that cyclic β-ketoesters are converted into corresponding 1,4-adducts in fine yields and with outstanding both diastereoselectivity and enantioselectivity (Table 3, Entries 1 and 2). Similarly, expected products were obtained with good selectivity when acyclic β-ketoesters were used, but the reactivity was decreased to some extent in this case (Table 3, Entry 3). What needs to be emphasized is that high diastereoselectivity and enantioselectivity can also be attained with trisubstituted carbon Michael donors, β-diketones as a class of particularly challenging substrates (Table 3, Entries 4 and 5).

An attractive feature of polymer-supported catalysts is that they can be easily removed at the end of reaction and recycled. The recycling performance of catalyst V in the presence of toluene as the reaction medium is displayed in Table 4. Encouragingly, catalyst V retains catalytic reactivity even after being extracted and reused for six times, accompanied by minor loss in stereoselectivity. We propose that the reaction in the presence of current synthetic polymer-supported catalysts proceeds via a dual activation model [31–33]. On one hand, as shown in Scheme 2, N-benzylmaleimide is assumed to interact with the hydroxyl group of V via hydrogen bond, thus enhancing the electrophilic character of the reacting carbon center. On the other hand, the enolic form of 1a is assumed to interact with the tertiary amine moiety, accompanied by subsequent deprotonation resulting in a highly nucleophilic enolate species. Although catalyst V has favorable selectivity, its reactivity is still unsatisfactory, possibly due to easy curling of chain segments of polymer-bound catalysts leading to shielding of active reaction centers.

In connection with the application of Michael addition in industry, we tested catalyst V on an enlarged scale of 20 mmol (Scheme 3). Similar satisfactory results were obtained as compared with those on a smaller scale (0.2 mmol), with isolated yield being 87%, the enantioselectivity and diastereoselectivity being 84% and 8:2, respectively. This indicates that the catalytic ability of catalyst V is unaffected by the scale, even though it is enlarged by 100 times.
4 Conclusion
A series of polymer-bound cinchona alkaloids was prepared by grafting quinine onto polymers such as mercaptomethyl-polystyrene and polystyrene. The resultant products were used as the catalysts for the asymmetric Michael reactions of 1,3-dicarbonyl compounds and N-benzylmaleimide. It has been found that catalyst V, obtained from the grafting reaction of quinine onto mercaptomethyl-polystyrene, shows good chiral catalytic activity in the above-mentioned asymmetric Michael reactions. The corresponding asymmetric Michael addition product 3, the first model of adjacent quaternary and tertiary stereocenters synthesized in the presence of a polymer-bound catalyst, has outstanding chiral selectivity of up to 86% ee. At the same time, immobilized alkaloid V possesses good recycled stereochemical reactivity.
References
Sibi MP, Manyem S (2000) Tetrahedron 56:8033
Jha SC, Joshi NN (2002) Arkivoc vii:167
Almasi DA, Alonso D, Na′jera C (2007) Tetrahedron Asymmetry 18:299
Vicario J, Badía D, Carrillo L (2007) Synthesis 2065
Tsogoeva SB (2007) Eur J Org Chem 1701
Singh P, Kumari K, Katyal A, Kalra R, Chandra R (2009) Catal Lett 127:119
Christoffers J, Mann A (2001) Angew Chem Int Ed 40:4591
Nojiri A, Kumagai N, Shibasaki M (2009) J Am Chem Soc 131:3779
Cozzi PG, Hilgraf R, Zimmermann N (2007) Eur J Org Chem 5969
Li H, Wang Y, Tang L, Wu F, Liu X, Guo C, Foxman BM, Deng L (2005) Angew Chem Int Ed 44:105
Kano T, Tanaka Y, Osawa K, Yurino T, Maruoka K (2009) Chem Commun 15:1956
Ogawa S, Yasui H, Tokunaga E, Nakamura S, Shibata N (2009) Chem Lett 38:1006
Shintani R, Tsutsumi Y, Nagaosa M, Nishimura T, Hayashi T (2009) J Am Chem Soc 131:13588
Marigo M, Schulte T, Franzén J, Jørgensen KA (2005) J Am Chem Soc 127:15710
Galzerano P, Bencivenni G, Pesciaioli F, Mazzanti A, Giannichi B, Sambri L, Bartoli G, Melchiorre P (2009) Chem Eur J 15:7846
Taylor MS, Zalatan DN, Lerchner AM, Jacobsen EN (2005) J Am Chem Soc 127:1313
Hamashima Y, Hotta D, Sodeoka M (2002) J Am Chem Soc 124:11240
Okino T, Hoashi Y, Furukawa T, Xu X, Takemoto Y (2005) J Am Chem Soc 127:119
Taylor MS, Jacobsen EN (2003) J Am Chem Soc 125:11204
Bartoli G, Bosco M, Carlone A, Cavalli A, Locatelli M, Mazzanti A, Ricci P, Sambri L, Melchiorre P (2006) Angew Chem Int Ed 45:4966
Bartoli G, Bosco M, Carlone A, Locatelli M, Melchiorre P, Sambri L (2005) Angew Chem Int Ed 44:6219
Thierry B, Audouard C, Plaquevent JC (2004) Synlett 856
Kreidler B, Baro A, Christoffers J (2005) Synlett 465
Alvarez R, Hourdin MA, Cavé C, d’Angelo J, Chaminade P (1999) Tetrahedron Lett 40:7091
Zhang Z, Wang Y, Wang Z, Hodge P (1999) React Funct Polym 41:37
Chinchilla R, Mazón P, Nájera C (2000) Tetrahedron Asymmetry 11:3277
Thierry B, Perrard T, Audouard C, Plaquevent JC (2001) Synthesis 11:1742
Hodge P, Khoshdel E, Warerhouse J (1983) J Chem Soc Perkin Trans I:2205
Dondoni A (2008) Angew Chem Int Ed 47:8995
Kobayashi N, Iwai K (1978) J Am Chem Soc 100:7071
Luo J, Xu LW, Hay R, Lu Y (2009) Org Lett 11:437
Tan B, Chua PJ, Zeng X (2008) Org Lett 10:3489
Tan B, Shi Z, Chua PJ, Zhong G (2008) Org Lett 10:3425
Acknowledgments
We are grateful to the Natural Science Foundation of Henan Province for the financial support (No. 2008A150003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, Y., Yang, G., Yang, L. et al. Construction of Adjacent Quaternary and Tertiary Stereocenters by Conjugate Addition Using Polymer-Supported Cinchona Alkaloids as Catalysts. Catal Lett 141, 481–488 (2011). https://doi.org/10.1007/s10562-010-0509-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-010-0509-7