Abstract
The quasicrystalline structures of alloys with nominal compositions of Al72Ni13.4Co14.6 and Al72.3Ni7.8Co19.8 were characterized by X-ray diffraction, transmission electron microscopy and scanning electron microscopy. For catalytic application, the solids were leached with an alkaline NaOH solution and tested at 373 K with two model reactions under atmospheric pressure: hydrogenation of crotonaldehyde and acetonitrile. The catalytic activities of these leached alloys were compared to that of a Raney nickel reference catalyst. Catalysts prepared from quasicrystals showed high catalytic activities and high selectivities towards butanal (in crotonaldehyde hydrogenation) and ethylamine (in acetonitrile hydrogenation).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Quasicrystals (QC) have well-ordered structures without periodicity, and exhibit noncrystallographic rotational symmetries (i.e. 5 and 10 symmetries). Since the historical discovery of the icosahedral phase in a rapidly solidified Al–Mn alloy by Shechtman [1], over 100 binary, ternary and quaternary alloys systems have been found in many quasicrystalline structural systems [2]. Significant efforts have been made for the study of alloy synthesis, structure and physical properties on Al–Cu–Fe and Al–Ni–Co systems [3–5].
Since the discovery in the late 80’s [6] of stable and highly ordered decagonal phases in a solidified Al70Ni15Co15 alloy, the Al–Ni–Co phase has attracted a lot the interest. The first realistic structural model of this phase, which can be described as a quasiperiodic arrangement of columnar clusters of atoms (called ‘decagonal atom clusters’) with a diameter of about 2 nm, was proposed from high-resolution electron microscopy (HREM) studies [7]. Two types of structures, which can be characterized as rhombic and pentagonal tiling in the arrangements of the decagonal clusters, were found as different temperature phases. Subsequently, many types of electron diffraction patterns were found for a wide range of compositions of the decagonal phase [8–11].
The potential application of quasicrystals for catalysis was first investigated by Nosaki et al. [12]. Like Raney nickel catalysts, after alkaline leaching, these solids show a relatively high specific surface area and a high density metallic sites for catalysis. The catalytic activities of samples prepared from various Al–Mn alloys with crystalline and quasicrystalline structures were compared for the methanol decomposition reaction. Results show that the highest hydrogen yield was obtained over the catalysts prepared from quasicrystalline form. Then, Tsai and coworkers [13–16] studied the catalytic properties of icosahedral quasicrystal systems from Al–Cu–Fe alloys with various compositions for the steam reforming reaction of methanol. Some fundamental studies of adsorption and/or reactivity of simple molecules (e.g., CO, CH3OH and H2) on the surface of quasicrystals such as Al–Pd–Mn [17, 18], Al–Ni–Co [19] and Ti–Zr–Ni [20] were also reported. In these studies, the objective was H2 production, but the similarity of QC alloys to Raney nickel suggests that excellent hydrogenation properties could be also obtained. In the present study, we will attempt to illustrate an alternative application of QC-based catalysts with two hydrogenation reactions.
Selective hydrogenation of α, β-unsaturated aldehydes to the corresponding alcohols is an important reaction used in the chemical industry to produce intermediates for fragrance, medicine and agricultural chemicals [21]. It is known that noble metal catalysts based on Pt, Ru or Rh promote preferentially the hydrogenation of the C=C bond. Many studies have been performed to improve the catalytic activity and the selectivity to unsaturated alcohols, including investigations of different supports [22, 23], metal-support interactions [24–26], addition of a second metal [27, 28] and promoter effects [29–31]. As an alternative to noble metal catalysts, the use of Ni is also studied for this group of hydrogenation reactions.
Nickel catalysts (especially Raney nickel catalyst) present a high activity for C=C bond hydrogenation reaction and low levels of doping with Co, Fe, Mn (less than 10%) promotes hydrogenation activity [32]. Addition of a small quantity of copper improves the selectivity for the C=O group hydrogenation and decreases the activity of catalysts [33, 34]. Cobalt supported and cobalt doped Raney catalysts possess high potential for C=O group hydrogenation and much work has been done to improve catalytic activity and selectivity in forming unsaturated alcohol, such as addition of Pt [35], B [36, 37] and Cl−or Br− [29].
Copper catalysts supported on Al2O3 and SiO2 were also studied for this hydrogenation reaction [30, 31]. Maximum selectivity in crotyl alcohol is about 10%, but the addition of Zn or S improves the selectivity. Alternative catalysts for this reaction can be prepared from quasicrystal alloys.
Another important hydrogenation reaction is the conversion of nitriles for the production of amines, which are widely used in the pharmaceutical, textile and plastic industries. The reaction is catalyzed by a variety of transition metals, especially by those from group eight. The nitriles can be hydrogenated either in liquid-phase or in the gas-phase. The metal used as catalyst determines the selectivity and activity. Thus, for the production of primary amines the nickel-based catalysts are commonly used [38]. In this case, the nature of the support strongly influences the selectivity and activity by its acid–basic properties: the acid sites are partly responsible for condensation reactions giving higher amines [39, 40], whereas the mixed oxides with strong basic properties increase the selectivity of the nickel catalysts to primary amines [41].
In the present work, we have investigated an Al–Ni–Co quasicrystalline system with different compositions. After leaching, their structural and catalytic properties for hydrogenation reaction of crotonaldehyde and acetonitrile were determined and compared with a Raney nickel reference catalyst.
2 Experimental
Alloys were prepared from pure elements (Al, Ni, Co) by atomization method at Saint Gobain [42] and composition determined by ICP (Spectro-D). TEM observation and electron diffraction were carried out on JEOL 2010 instrument equipped with an EDS Link Isis microanalysis system. Morphology was characterized by SEM with a Hitachi S800 microscope. The XRD patterns were recorded using a Bruker D5005 diffractometer with Cu Kα radiation. In situ reduction, from room temperature and up to 973 K, patterns was recorded using a Panalytical X’Pert Pro MPD diffractometer (X’Celerator detector) equipped with an Anton Paar XRK 900 reactor chamber. The phases were identified using the Powder Diffraction File (PDF) database (JCPDS, International Centre for Diffraction Data).
Specific surface areas were measured by nitrogen physisorption at 77 K. X-ray photoelectron spectroscopy (XPS) experiments were performed in a KRATOS Axis Ultra DLD spectrometer using monochromated Al-Kα radiation source at 1486.6 eV.
Before catalytic testing, the powder samples were leached in 20 wt.% NaOH aqueous solution, similarly to the alkaline treatment of Raney catalysts [43, 44]. Subsequently, the samples were kept in the alkaline solution for 2 h at ambient temperature and then kept 1 h in the boiling solution, after which they were decanted and washed with distilled water until no alkali was detected in the filtrate. The catalysts were either dried in vacuum at 373 K for 4 h or kept in water. The purpose of the drying procedure under vacuum is to oxidize nickel and cobalt atoms on the catalyst surface and to avoid the sintering of catalyst during contact with air. Reactivation of the catalyst is then performed by in situ reduction.
The Temperature Programmed Reduction analyses were performed in a conventional apparatus, using a micro GC equipped with a thermal conductivity detector. The reduction was carried out from room temperature up to 873 K (10 K/min), under a 50 mL/min flow rate of 5% hydrogen/nitrogen gas mixture [45].
The extent of reduction (R) of nickel (or cobalt) was determined by the magnetization method. The saturation magnetization of catalysts was measured with an electromagnet providing moderate fields (20904 Oe) at 300 K by removing the sample rapidly from the coil. The relative accuracy on the magnetization measurements was 10−3. The formula applied is \( R = {\frac{{M_{\text{S}} }}{{m\sigma_{S} }}}100 \) where M s is saturation magnetization measured, σ S is specific magnetization of nickel (or cobalt) and m is total mass of nickel (or cobalt). For the AlNiCo catalysts, we calculated the average specific magnetization of two metals (nickel and cobalt) after correction of the effect of residual Al in catalysts upon specific magnetization of nickel reported by Martin et al. [46] and upon specific magnetization of cobalt reported by Richard [47].
The crotonaldehyde hydrogenation was carried out in a tubular fixed bed reactor at 373 K and atmospheric pressure. Before reaction, the dried catalyst samples were reduced in situ under a flow of 50% hydrogen +50% nitrogen at 623 K for 2 h. For samples kept in water, in situ removal of water before reaction is necessary under a flow of 50% H2 in N2 gas mixture at 373 K for 2 h. Weight of catalyst kept in water was determined by picnometry method. The value of the density of the solid used for this determination is 4.5 g/cm3.
Crotonaldehyde or acetonitrile (>99% purity, Aldrich) was introduced in a saturator-condenser system submerged in a cryostat bath at 273 K and flushed with H2. Reaction products and unreacted crotonaldehyde or unreacted acetonitrile were analyzed online by a gas chromatograph (HP 4890A) equipped with a flame ionization detector and a Chrompack CP-SK-SCB column (length 25 m, diameter 0.32 mm).
Reaction rate of crotonaldehyde and acetonitrile is defined as the number of moles of crotonaldehyde and acetonitrile consumed, respectively, per second divided by the weight of catalyst (g). Conversion to a certain product is defined as the fraction of the crotonaldehyde or an acetonitrile feed converted into that product. A Raney Ni (Prolabo) catalyst was leached in a similar manner as the one described above and used as a reference for the comparison of catalytic performances. For both catalytic reactions, the results were reproducible within 5%.
3 Results and Discussion
3.1 Characterization
The quasicrystalline structure of alloys with nominal compositions of Al72Ni13.4Co14.6 (referred to as AlNiCo1) and Al72.3Ni7.8Co19.8 (referred to as AlNiCo2) which are used in this study fall in the decagonal zone of the ternary Al–Ni–Co phase diagram proposed by Yurechko et al. [48]. One is situated at the left border and the other in the middle of this decagonal domain.
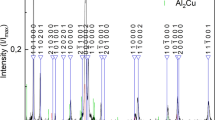
Figure 1 shows the X-ray powder diffraction patterns of two alloys based on Ni and Co. Their diffraction patterns look similar and correspond to that of quasicrystalline structure of ternary Al70Co15Ni15 (PDF 47-1407) and corresponds to type I phase. Morphology of the two alloys was characterized by SEM and shown in Fig. 2a, b. Both alloys present a spherical shape (particles with an average diameter of about 10 μm). This morphology is particularly suitable for catalytic applications. Since there are no apparent differences between the two Al–Ni–Co alloys, only AlNiCo1 alloy was further characterized using electron diffraction and HRTEM techniques.
The electron diffraction patterns of the alloy confirm the presence of a decagonal phase (Fig. 3). We can observe several rings of spots of ten-fold symmetry around the center of the diffraction image. It is close to most typical diffraction patterns of AlNiCo systems with decagonal quasicrystalline structure [49, 50]. HRTEM image illustrates also the Penrose tiling of the superstructure Fig. 4. The presence of a thin layer of Al2O3 (below 2 nm) at the surface of the grains was also revealed by HRTEM.
Upon in situ reduction up to 973 K, AlNiCo1 does not present any structural modification. On the contrary, AlNiCo2 alloy with initial decagonal structure (PDF 47-1407) was partly modified into another decagonal phase Al70Ni10Co20 (PDF 50-0963) after heat treatment at 973 K under hydrogen flow. The structural transformation of AlNiCo2 alloy under hydrogen flow was followed in situ by XRD temperature reduction (see Fig. 5). From ambient temperature up to 823 K, the intensity of characteristic peaks does not change, but their positions are slightly shifted due to lattice expansion. From 848 K, a decrease of the intensity of the diffraction peaks characteristic of the decagonal phase Al70Ni15Co15 (PDF 47-1407) is observed. Simultaneously, new peaks characteristic of another decagonal phase of Al70Ni10Co20 (PDF 50-0963) appear and remain up to 973 K. This corresponds to type I↔S 1 phase transition [10].
The textural properties of the alloys are given in Table 1. The specific surface area of the initial particles is below 1 m2/g, similar to Raney nickel alloy.
After alkali leaching, the metallic catalysts present specific surface areas in the range of 30–50 m2/g. Alkali leaching considerably reduces Al content with only a few % of Al remaining (see composition after leaching in Table 1). After alkaline leaching, alloys can not retain their initial structures; this treatment appears to have completely modified the structure of alloys into amorphous form and weak contributions of Ni and Co metallic cubic phases are detected. The apparent crystallite size of the metallic particles estimated from XRD patterns is also listed in this Table 1. According to this technique, metal particles are well dispersed (below 4 nm) similar to pure Raney Ni catalysts. The presence of Al(OH)3 was observed with low intensity (Fig. 6). The modifications induced by alkali leaching are similar to those reported by Fouilloux [39] on Raney nickel alloys.
The morphological transformation of AlNiCo1 catalysts (and AlNiCo2 is similar and not reported here) prepared by alkaline leaching was monitored by SEM and is shown in Fig. 2c. The initial spherical shape of the particles is partially kept, but the particles appear to be more porous with a rougher surface.
The TPR profiles of dried samples are shown in Fig. 7. The first peak of hydrogen consumption in the 510–540 K range was assigned to reduction of the NiO, CoO. A low intensity peak observed at 600 K for the AlNiCo2 sample is characteristic of the reduction of the Co3O4 phase [51, 52]. This peak is not clearly observed for the AlNiCo1 sample because of its low intensity and its overlap with the fist peak. Before catalytic reaction, we have chosen the in situ reduction at 623 K under 50% H2 in nitrogen flow for all catalysts dried in vacuum.
Magnetic measurement is a highly accurate technique for the determination of the extent of reduction of Ni or Co metal catalysts [53]. In order to see if the passivation/reduction procedure causes particle sintering and if it possible to recover the reduced state of the sample kept in water, the magnetization curve of Raney catalyst was acquired at various stages (kept in water, passivated and reduced). We can observe (Fig. 8) that in situ reduction of the passivated sample allows recovery of the magnetization of the sample kept in water. From the magnetization curves, we can determine the extent of reduction of the metallic phases. Table 2 summarizes these results. As mentioned in the experimental part, even for Raney catalyst, the system is rather complex due to the presence of remaining Al. In the QC-based catalyst, this is even more complex due to the presence of another magnetic phase, Co. The assumptions used for the determination are presented in the experimental part. During drying in vacuum, catalysts have been partially oxidized; such passivation prevents sintering in contact with air. Nickel in the surface of Raney nickel catalyst has been oxidized about 50%. But the presence of cobalt in AlNiCo catalysts seems to limit the extent of oxidation in comparison to Raney nickel, with remaining metallic contribution in the range of 60%. When the passivated sample is reduced in situ, a degree of reduction close to 100% is obtained. Keeping the samples in neutral water leads to a partial oxidation occurring at the surface of the catalysts. Thus, the degree of reduction remains in the range of 92–96%. A more detailed characterization of this surface oxidation is given by the XPS analysis. Figure 9 shows the XPS spectra of Ni 2p (Fig. 9a) and Co 2p (Fig. 9b) in AlNiCo catalysts (dried under vacuum, kept in water and kept in NaOH preparation solution). The high resolution of the spectrometer allows distinguishing the two contributions of metallic and oxide state of the two elements. For the AlNiCo1 sample dried under vacuum, a peak Ni 2p3/2 at 855.8 eV characteristic of the nickel oxide is detected with a high intensity which could be attributed to NiAl2O4 or Ni(OH)2 states. A peak Co 2p3/2 at 781 eV is characteristic of cobalt oxide under the form of CoAl2O4 or Co(OH)2. These two peaks of Ni and Co oxide contributions cannot be detected in the case of AlNiCo catalysts kept in NaOH solution and spectra correspond to metallic references [54].
The contribution of each kind of species can be evaluated by decomposition of the peak as reported in Table 3. This result is similar to the observation of other authors on Raney nickel who indicated that nickel on the surface of Raney nickel catalyst could be oxidized about 30–40% when kept in water [55, 56]. Surface oxidation of catalysts passivated and kept under neutral water corresponds respectively to more than 80% and less than 70%. In AlNiCo1 sample, Co atoms are slightly more oxidized than Ni ones.
3.2 Catalytic Tests
3.2.1 Crotonaldehyde Hydrogenation
Catalytic activities in the conversion of crotonaldehyde, measured at 373 K, are shown in Table 4. The rate of conversion (rcroton) and selectivity (crotyl alcohol/butanal ratio) were determined at about 15% conversion of crotonaldehyde by changing the contact time. As compared to Raney nickel reference catalysts, QC catalysts exhibit comparable or higher activities. Under these low conversion conditions, catalysts only produced butanal.
Catalytic activity of catalysts kept in water (no reactivation by in situ reduction at 623 K before catalytic reaction) is always higher than that of catalysts dried in vacuum, which can be explained by a slight difference of surface composition of the catalysts and/or a small sintering during the in situ reduction.
The presence of cobalt in AlNiCo catalysts has not significantly improved the activity of the catalysts. The stability of QC catalysts is comparable or even better that of Raney nickel reference catalyst (see Fig. 10).
Variation of crotonaldehyde conversion and selectivity of products by contact time (hydrogen flow and/or catalyst mass) are shown in Fig. 11. The distribution of products in a wide range of conversions does not change significantly as compared to Raney nickel catalyst even if cobalt is less active than nickel in this hydrogenation reaction. As compared to the literature, we do not observe any impact of the presence of Co on the selectivity [35, 36]. The preparation method used for the synthesis of QC compositions involves the manufacture of a true alloy between Ni and Co whereas conventional impregnated catalysts may not present such close combination of the two metals. Thus, with a true alloy composition, no electronic effect which controls the adsorption mode of α, β-unsaturated aldehydes on the surface and which might favor the selectivity towards crotyl alcohol is observed. One can assume that the selectivity observed on Co based catalysts in previous studies originates from the contribution of segregated Co particles [35, 36].
Up to 80% conversion, selectivity into butanal is higher than 90%. Above this conversion level, butanal starts to be transformed into butanol. Light and heavy compounds were detected with concentrations lower than 0.5% because of cracking and condensation reactions of crotonaldehyde and/or products.
The product selectivity of catalysts based on Ni, Co is thus explained by the simplified mechanism of two consecutive hydrogenation reactions Scheme 1.
The presence of residual Al in catalysts with content of 3.5–9% plays an important role in the performance of Raney type nickel catalysts, because it can act as an electron donor to nickel, rendering the d-band of nickel less electron deficient, and thus influencing the absorption of the reacting species [42]. So, the remaining metallic Al has a pronounced effect on the magnetization signal of catalysts. Moreover, by a systematic theoretical calculation, Delbecq et al. [57] suggested that the main attractive effect in the adsorption of aldehyde on surface is the back-donation from the metal orbitals into the π*CO orbital. In this respect, the electron donation from Al to Ni can enhance such back-donation and consequently favour the bonding using the carbonyl group. But in our case, we have not observed such an effect of the residual Al upon the selectivity of crotyl alcohol.
3.2.2 Acetonitrile Hydrogenation
Similarly, catalytic activities in the conversion of acetonitrile hydrogenation were measured at 373 K, as shown in Table 5. The following reaction scheme proposed by Li et al. [58] illustrates the various pathways of the reaction Scheme 2.
Acetonitrile hydrogenation reaction scheme [54]
Only dried catalysts were studied in that case. The rate of conversion (racetonitrile) was determined at about 15% conversion of acetonitrile by changing the contact time. Selectivity was checked for all conversion levels tested and are compared at total conversion in Table 5. AlNiCo1 catalysts exhibit slightly higher activities than Raney nickel reference catalysts. Variation of acetonitrile conversion and selectivity to products by contact time (hydrogen flow and/or catalyst mass) are shown in Fig. 12 and can be interpreted as parallel reactions, the main product being ethylamine and two side products being diethyl and triethylamine. The two catalysts based on Ni–Co alloys present the same behaviour. At 100% conversion, the main product is ethylamine. The products distribution shows a high selectivity towards ethylamine (97–98%) and the presence of diethylamine (3–2%) as by products (see Table 5; Fig. 12). Raney nickel selectivity towards ethylamine is smaller (89%) and diethylamine and triethyl amines are observed in larger amounts (9 and 1% respectively). Traces of heavy products were detected mainly on the Raney nickel catalysts. Thus, the catalysts prepared from QC NiCo alloys are more selective than the Raney catalyst.
4 Conclusions
This work illustrates the potential application of quasicrystals as catalyst precursors. Decagonal structure of two AlNiCo alloys has been investigated by using SEM, HRTEM and electron diffraction and in situ XRD. These alloys, after alkaline treatment, provide NiCo dispersed metal catalysts in a skeletal form with high degree of reduction. By alkali leaching, these quasicrystalline alloys can be used as precursors for highly active hydrogenation catalysts comparable to or more active than a reference Raney Ni catalyst. This type of catalysts based on nickel and cobalt is very active in hydrogenation reactions of crotonaldehyde and acetonitrile. For the acetonitrile reaction, the QC catalysts present similar activities to Raney nickel catalysts, but exhibit a higher selectivity towards the desired product.
References
Shechtman D, Blech IA, Gratias D, Cahn JW (1984) Phys Rev Lett 53:1951
Tsai AP, Stadnik ZM (1999) Physical properties of quasicrystals, Springer series in solid-state science, vol 126. Springer, Berlin, p 5
Wang L, Tan Z, Zhang J, Zhou Q, Dong C (1999) Thermochimica Acta 331:21
Rakchoun IV, Menushenkov AP, Chaitoura DS, Klementev KV (2005) Nucl Inst Meth Phys Res A 543:208
Steurer W (2004) J non-cryst Sol 334 & 335:137
Tsai AP (2003) Acc Chem Res 36:300
Hiraga K, Lincoln FJ, Sun W (1991) Mater Trans Jpn Inst Metals 32:308
Edagawa K, Ichihara M, Takeuchi S (1992) Phil Mag Lett 66:697
Edagawa K, Sawa H, Takeuchi S (1994) Phil Mag Lett 69:227
Ritsch S, Beeli C, Nissen HU, Luck R (1995) Phil Mag A 71:671
Grushko B, Holland-Mortz D, Wittmann R, Willde G (1998) J Alloys Compd 280:215
Nosaki K, Masumoto T, Inoue K, Yamaguchi T (1998) US patent N° 5800638
Yoshimura M, Tsai AP (2002) J Alloys Compd 342:451
Tsai AP, Yoshimura M (2001) Appl Catal 214:237
Tanabe T, Kameoka S, Tsai AP (2006) Catal Today 111:153
Yamasaki M, Tsai AP (2002) J Alloys Compd 342:469
Jenks CJ, Thiel PA (1998) J Mol Cat A Chemical 131:301
Jenks CJ, Lofrasso TA, Thiel PA (1998) J Am Chem Soc 120:12668
McGrath R, Ledieu J, Cox EJ, Jenks CJ, Lofrasso TA (2002) J Alloys Compd 342:432
Sadoc A, Majzoub EH, Huette WT, Kelton KF (2003) J Alloys Compd 356 & 357:96
Gallezot P, Richard D (1998) Catal Rev Sci Eng 40:81126
Kluson P, Cerreny L, Had J (1994) Catal Lett 23:299
Ammari F, Lamotte J, Touronde R (2004) J Catal 221:32
Vannice MA, Sen B (1989) J Catal 115:65
Vannice MA, Poondi D (1997) J Catal 169:166
Wismeijer A, Kieboom APG, van Bekkum H (1986) Appl Catal 25:181
Abid M, Ehret G, Touroude R (2001) Appl Catal A 217:219
Concepcion P, Corma A, Silvestre-Albero J, Franco V, Chane-Ching Y (2004) J Am Chem Soc 126:5523
Kurokawa H, Mori K, Yoshida K, Ohshima M, Sugiyama K, Miura H (2005) Catal Comm 6(12):766
Hutchings GJ, King F, Okoye IP, Rochester CH (1992) Appl Catal A 83:L7
Rodrigues EL, Marchi AJ, Apesteguia CR, Bueno JMC (2005) Appl Cata A 294:197
Montromery SR (1981) Catalysis of organic reactions (Ed. W.R. Moser), Dekker, New York, USA, p 383
Noller H, Lin WM (1984) J Catal 85:25
Raab CG, Lercher JA (1992) J Mol Catal 75:71
Ando C, Kurokawa H, Miura H (1999) Appl Catal A 185:L181
Li H, Chen X, Wang M, Xu Y (2002) Appl Catal A 225:117
Pei Y, Hu H, Fang J, Qiao M, Dai W, Fan K, Li H (2004) J Mol Catal A Chemical 211:243
Gluhoi AC, Marginean P, Stanescu U (2005) Appl Catal A 294:208
Medina F, Dutartre R, Tichit D, Coq B, Dung NT, Salagre P, Sueiras JE (1997) J Mol Cata A Chemical 119:201
Medina F, Tichit D, Coq B, Vaccari A, Dung NT (1997) J Catal 167:142
Coq B, Tichit D, Ribet S (2000) J Catal 189(1):117
Janot C, Dubois JM (1998) Les quasicristaux, matière à paradoxe, EDP Sciences, p 169
Fouilloux P (1983) Appl Catal 8:1
Mellor JR, Covflle NJ, Sofianos AC, Copperthwaite RG (1997) Appl Catal A 164:171
Marta CN, Carvalho A, Passos FB, Schmal M (2002) Appl Catal A 232:147
Martin GA, Fouilloux P (1975) J Catal 38:231
Richard MB, Ferromagnetism, Second trinting, Van Nostrand D (1951) Company, Inc p 284
Yurechko M, Grushko B, Velikanova TY, Urban K (2004) J Alloys Compd 367:20
Hiraga K, Ohsuna T, Nishimura S (2000) Phil Mag Lett 80:653
Hiraga K, Ohsuna T, Nishimura S (2001) Phil Mag Lett 81:109
Sexton A, Hughes AE, Turney TW (1986) J Catal 97:390
Rodrigues EL, Bueno JMC (2004) Appl Catal A General 257:201
Dalmon JA (1994) Catalyst characterization: physical techniques for solid material, edited by Boris Imelik and Laces C. Vedrine, Plenum Press, New York p 585
Moulder JF, Strckle WF, Sobol PE, Bomben KD (1992) Hand book of X-ray photoelectron spectroscopy, Edited vay J. Chastain, Perkin-Elmer Corporation p 82
Lei H, Song Z, Tan D, Bao X, Mu B, Zong E, Min E (2001) Appl Catal A 214:69
Hoffer BW, Crezee E, Devred F, Mooijman PRM, Sloof WG, Kooyman PJ, Langevel AD, Kapteijn F, Moulijn JA (2003) Appl Catal A 253:437
Delbecq F, Sautet P (1995) J Catal 152:217
Li H, Wu Y, Luo H, Wang M, Xu Y (2003) J Catal 214:15
Acknowledgments
The authors thank St. Gobain CREE for providing the samples and the financial support. Education and Training Ministry of Vietnam is also acknowledged for providing a grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ngoc, B.P., Geantet, C., Dalmon, J.A. et al. Quasicrystalline Structures as Catalyst Precursors for Hydrogenation Reactions. Catal Lett 131, 59–69 (2009). https://doi.org/10.1007/s10562-009-0018-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-009-0018-8














 ,
Butanol
,
Butanol  , Crotyl alcohol
, Crotyl alcohol  , Heavy products
, Heavy products 



 , Dimethylamine
, Dimethylamine  , Triethylamine
, Triethylamine  , Heavy products
, Heavy products 