Abstract
Gold nanoparticles are deposited on potassium titanate nanowires and used as heterogeneous catalysts in the aerobic oxidation of benzyl alcohol in methanol to methyl benzoate at ambient conditions. The presence of a catalytic amount of base promotes the reaction and the formation of free benzoic acid during the reaction poisons the catalyst. The activity however, of the catalyst can be restored again by addition of base.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In organic chemistry, the selective oxidation of alcohols is one of the most important reactions and it is applied on an industrial scale in the production of numerous base chemicals down to laboratory scale in the production of various specialty and fine chemicals [1–5]. Recently, there has been a considerable focus on aerobic oxidations, using air or pure oxygen as the oxidant rather than e.g., the classical metal oxides. The main reason for this new approach can be attributed to the low cost of oxygen and the fact that oxygen is a “green” oxidant leading to water as the only by-product. For many years, metallic gold was considered to be too inactive to be useful in heterogeneous catalysis. However, this changed with the discovery that gold nanoparticles can indeed be very active in oxidation reactions as reported first by Haruta et al. for low temperature aerobic oxidation of carbon monoxide [6]. Following the discovery by Haruta et al., a variety of aerobic oxidation reactions have been reported [7, 8]. For instance, gold nanoparticles were used in the oxidation of alcohols to aldehydes [9], carboxylic acids [10–14] or esters [15, 16], in the oxidation of aldehydes to esters [17] or acids [18] in epoxidations of olefins [19, 20], and in the oxidation of amines to amides [21]. Although Haruta et al. [6] were able to achieve low temperature aerobic oxidation of carbon monoxide, most aerobic oxidations using gold catalysts require temperatures and oxygen pressures well above ambient conditions. Often this results in a limited life-time of the gold catalyst. To stabilize the gold nanoparticles, a number of different supports have been tested as support materials. The majority of these supports are metal oxide nanoparticles that are stable at high temperatures and chemically inert. The most commonly used metal oxide support for gold catalysts is TiO2. Recently, a new family of materials with some similarity to TiO2 was reported. They include nanotubes and nanowires of alkali metal titanates prepared simply by hydrothermal treatments of TiO2 in the presence of an alkali metal base [22–25]. These materials are very easy to produce, inexpensive, stable and most importantly, they typically have higher surface areas than those of the starting TiO2 nanoparticles. All these properties appear to make them very promising support materials in heterogeneous catalysis. Here, we report the use of high-surface area potassium titanates, K2Ti6O13, nanowires as a support material for gold nanoparticles prepared by a simple precipitation. Furthermore, we show that the obtained gold catalyst is able to selectively oxidize benzyl alcohol in methanol into the corresponding methyl esters, via the aldehyde, at room temperature and atmospheric pressure. Finally, we demonstrate that the presence of a catalytic amount of base promotes the reaction and the formation of free benzoic acid during the reaction poisons the catalyst. However, the activity of the catalyst can be restored again by addition of base. In selective oxidations of alcohols over gold catalysts, the effect of having acids or bases present is critical. For instance, in the oxidation of aqueous ethanol to produce acetic acid, no addition of base is needed but for other oxidations in water normally a stoichiometric amount of base is required [12–14]. If instead methanol is used as a solvent, only a catalytic amount of base is needed. Thus, the role of base and acid in these reactions has remained elusive, and here we will try to understand this phenomenon in more detail.
Previously, it was shown by Du et al. that a hydrothermal treatment of TiO2 with KOH leads to the formation of nanowires of potassium titanates, K2Ti6O13 [26]. These nanowires have a different structure than the more known titanate nanotubes, obtained by using NaOH instead of KOH [22–25]. Very recently, protonic titanate nanotubes made by ion-exchanges of sodium titanate nanotubes were used as a support material for gold nanoparticles and tested as a catalyst in the aerobic oxidation of CO [27, 28].
2 Experimental
2.1 Synthesis of Gold Catalysts
Potassium titanates, K2Ti6O13 nanowires were prepared by hydrothermal treatment of TiO2 with KOH and used to support gold nanoparticles. In a typical synthesis of the potassium titanate nanowire support, commercial TiO2 anatase nanoparticles (1.92 g) were suspended in aqueous KOH (160 mL) of different concentrations, followed by hydrothermal treatment at 150 °C in a stainless Teflon-lined autoclave [26, 29]. The resulting powders were washed with large amounts of distilled water until neutral pH was achieved in the washing water. During the hydrothermal treatment with KOH, the TiO2 nanoparticles are slowly transformed into K2Ti6O13 nanowires.
Gold nanoparticles were deposited on the nanowires by deposition-precipitation using HAuCl4 ⋅ 3H2O as the gold precursor to yield a total catalyst loading of 1 wt% of gold [30]. The precipitated gold oxides were reduced using H2 in N2 at 300 °C for 2 h with at heating ramp of 5 °C/min. This results in gold nanoparticles with an averages size of 4–6 nm.
2.2 Catalytic Experiments
The catalytic reactions were carried out in a glass flask fitted with a condenser connected to an O2 cylinder. A mixture of benzyl alcohol and methanol (1/30 molar ratio) was charged to a 25-mL round-bottomed flask equipped with a condenser together with the catalyst (0.1 mol% Au). In experiments with KOH as the added base, the KOH was first dissolved in the methanol. Samples were analyzed by GC and GC-MS; the amounts of substrates and products were quantified using anisol as an internal standard.
3 Results and Discussion
Figure 1a shows representative high-resolution transmission electron microscope (TEM) images of the prepared K2Ti6O13 nanowires. The images were obtained by means of a JEM 2000FX microscope operated at an accelerating voltage of 300 kV. The nanowires have a diameter of about 5–10 nm and an average length of around 500 nm. Figure 1b shows a gold nanoparticle with a size of ca. 4 nm supported on a potassium titanate nanowire. The images with gold supported on nanowires were obtained using a Philips CM300 FEG microscope at 300 kV.
Table 1 shows the BET (Brunauer–Emmett–Teller) surface areas of the samples after hydrothermal treatment with different concentrations of KOH. It is seen that during the treatment of the TiO2 nanoparticles (entry 1) with KOH to form K2Ti6O13 nanowires, the surface area of the obtained material gradually increased. For example, for the high concentrations of KOH (entry 5 and 6) the transformation into K2Ti6O13 results in significantly higher surface areas, almost twice as large as that of the starting TiO2 nanoparticles. For the lower concentrations of KOH, the transformation of TiO2 into K2Ti6O13 is not complete as evidenced by X-ray powder diffraction. In comparison, a similar hydrothermal treatment with pure water and no base (entry 2) leads to a significant decrease of the surface area to 88 m2/g.
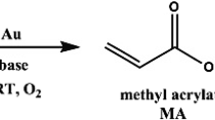
The rate-determining step in aerobic oxidations of alcohols to the corresponding carboxylic acids and its derivatives is assumed to be the oxidation of the alcohol to the aldehyde [31, 32]. This is accordance with the observation that the formation of the carboxylic acid derivative from the aldehydes proceeds very fast, in fact with a reasonable rate even below −70 °C [17]. Therefore, to achieve full conversion in the oxidation of alcohols, elevated reaction temperature is usually needed [12–14, 21, 31–35]. However, recently Miyamura et al. showed by using an exotic gold catalyst (polymer-incarcerated gold nanoparticles) it is indeed possible to achieve aerobic oxidation of mainly secondary alcohols to ketones at ambient conditions by using a stoichiometric excess of base [36]. In this work, we show that it is possible to use gold nanoparticles supported on the K2Ti6O13 nanowires for the selective oxidation of benzyl alcohol in methanol to the methyl ester at room temperature and atmospheric pressure with much less base (Scheme 1).
In Fig. 2, the yield of methyl benzoate is shown as a function of reaction time using 1 wt% Au supported on either K2Ti6O13 or TiO2 [37]. It is clearly seen in the figure that it is possible to oxidize benzyl alcohol at room temperature and ~1 bar O2 over the gold catalysts. When K2Ti6O13 was used as a support, a yield of 13% was obtained after 48 h without any added base. After several days, a yield of around 18% was reached. Furthermore, it is seen that the addition of a catalytic amount of base to the reaction mixture increase the reaction rate and the extent of reaction dramatically, resulting in >99% conversion after 24 h and a yield of methyl benzoate of about 93%. The main by-products in the reactions are benzoic acid, benzaldehyde and benzyl benzoate. The intermediate benzaldehyde is present in small amounts during the entire reaction, but the concentration decreases as the amount of benzyl alcohol decreases. The amount of benzyl benzoate increases during the first 2 h of the reaction but when the concentration of benzyl alcohol decreases so does the yield of benzyl benzoate. Figure 2 also shows how the reaction is catalyzed by gold supported on the unmodified TiO2 nanoparticles. However, the yield of methyl benzoate is consistently lower in comparison with those obtained using gold supported on K2Ti6O13. In the case of no added base and Au/TiO2, the reaction proceeded very slowly. A simple explanation is that the K2Ti6O13 nanowires are slightly more basic than the unmodified TiO2. Therefore, we decided to add a catalytic amount of solid base, MgO (15 mol%), to the reactions mixture using the traditional Au/TiO2 catalyst, and this resulted in full conversion of benzyl alcohol with a yield of methyl benzoate of 86% after 8 days. This shows that also solid bases are able to promote the reaction and this is clearly important for practical purposes since it facilitates isolation of the product (and the added base) compared with using a soluble base.
Generally, reactions performed without any base added seems to reach a certain level of conversion without approaching full conversion, cf. Fig. 2. If at this point, base is added to reaction mixture, the reaction accelerates dramatically and essentially proceeds to full conversion (>99) with yields comparable to those obtained when the reaction was started in the presence of a catalytic amount of base, Fig. 3.
a 0 mol% of KOH, after 115 h addition of 15 mol% of KOH. b 0 mol% KOH, after 2 h addition of 1 mol% benzoic acid resulting in deactivation of the catalyst. After 45 h the catalyst is reactivated by addition of 15 mol% KOH. c A deactivated catalyst is used without KOH. After 24 h, 15 mol% is added to reactivated the catalyst. All reaction ends up in full conversion (>99) of benzyl alcohol and similar yields (90–95%) of methyl benzoate as starting the reactions with base
Figure 3 illustrates the effect of adding a catalytic amount of base after 115 h to the reaction performed without any KOH using Au/K2Ti6O13 as catalyst. We suspected that the reaction stopped due to the formation of benzoic acid because benzoic acid either directly deactivated the catalyst and/or lowered pH in the solution. To determine the influence from the formation of benzoic acid on the reaction mixtures, benzoic acid (1 mol% relative to benzyl alcohol) was added after 2 h to a reaction mixture using the Au/K2Ti6O13 catalyst and without any base added. As seen in Fig. 3, the reaction rate drops dramatically upon addition of benzoic acid. After 45 h, the reaction was restarted by adding base, and full conversion of benzyl alcohol was observed almost immediately with a yield of methyl benzoate that was comparable to that achieved in the reaction performed without addition of benzoic acid. To further clarify how the presence of benzoic acid influences the catalyst performance, a catalyst, Au/K2Ti6O13, that was used for 4 days without any base added was filtered off, was washed carefully with large amounts of methanol and dried. This catalyst was then added into a new reaction mixture. Figure 3 shows how it exhibited essentially no activity. After 24 h, base was added to the reaction mixture and the catalytic activity was completely restored and a full conversion of benzyl alcohol was observed. This suggests that during the reaction, the catalyst is slowly deactivated by benzoic acid and it requires base to remove it from the catalyst surface.
4 Conclusions
To summarize, potassium titanates K2Ti6O13 was synthesized with high surface area and used as a support for the gold nanoparticles. The resulting gold catalysts were used to catalyze the aerobic oxidation of benzyl alcohol in methanol to the methyl benzoate via the aldehyde at room temperature and atmospheric pressure. The titanate nanowires are slightly basic and therefore shows improved performance. The significance of adding solid or soluble base, and free benzoic acid were studied. It is suggested that the presence of free carboxylic acid poisons the Au catalysts. In water, the acid can be removed by adding stoichiometric amounts of base. In methanol, less base is required since the dominant product is the methyl ester rather than the acid, and for ethanol oxidation, the reaction is conducted at a sufficiently high temperature that acetic acid is not blocking all available sites.
References
Franz G, Sheldon RA (2005) Ullmann’s encyclopedia of industrial chemistry. Weinheim, Wiley-VCH
Weissermel K, Arpe H-J (1997) Industrial organic chemistry, 3rd edn. VCH Verlagsgesellschaft, Weinheim
Besson M, Gallezot P (2000) Catal Today 57:127
Mallat T, Baiker A (1994) Catal Today 19:247
Ishida T, Haruta M (2007) Angew Chem Int Ed 46:7154
Haruta M, Yamada N, Kobayashi T, Iilima S (1989) J Catal 115:301
Corma A, Garcia H (2008) Chem Soc Rev 37:2096
Haruta M (2004) Gold Bull 37:267
Enache DI, Edwards JI, Landon P, Solsona-Espriu B, Carley AF, Herzing AA, Watanabe M, Kiely CJ, Knight DW, Hutchings GJ (2006) Science 311:362
Abad A, Concepción P, Corma A, Garcia H (2005) Angew Chem Int Ed 44:4066
Prati L, Rossi M (1998) J Catal 176:552
Christensen CH, Jørgensen B, Rass-Hansen J, Egeblad K, Madsen R, Klitgaard SK, Hansen MR, Andersen HC, Riisager A (2005) Angew Chem Int Ed 44:4648
Mallat T, Baiker A (2004) Chem Rev 104:3037
Carrettin S, McMorn P, Johnston P, Griffin K, Kiely CJ, Hutchings GJ (2003) Phys Chem Chem Phys 5:1329
Hayashi T, Inagaki T, Itayama N, Baba H (2006) Catal Today 117:210
Nielsen IS, Taarning E, Egeblad K, Madsen R, Christensen CH (2007) Catal Lett 116:35
Marsden C, Taarning E, Hansen D, Johansen L, Klitgaard SK, Egeblad K, Christensen CH (2008) Green Chem 10:168
Corma A, Domine ME (2005) Chem Commun 32:4042
Hayashi T, Tanaka K, Haruta M (1998) J Catal 178:566
Haruta M (2001) Appl Catal A 222:427
Klitgaard SK, Egeblad K, Mentzel UV, Popov AG, Jensen T, Taarning E, Nielsen IS, Christensen CH (2008) Green Chem 10:419
Zhang S, Chen Q, Peng L-M (2005) Phys Rev B 71:014104
Chen Q, Du GH, Zhanga S, Penga L-M (2002) Acta Cryst B58:587
Kolen’ko YV et al (2006) J Phys Chem B 110:4030
Du GH, Chen Q, Peng L-M (2001) Appl Phys Lett 79:3702
Du GH, Chen Q, Han PD, Yu Y, Peng L-M (2003) Phys Rev B 67:035323
Zhu B, Li K, Feng Y, Zhang S, Wu S, Huang W (2007) Catal Lett 118:55
Jiang J, Gao Q, Chen Z (2008) J Mol Catal A: Chem 280:233
Miao L, Ina Y, Tanemura S, Jiang T, Tanemura M, Kaneko K, Toh S, Mori Y (2007) Surf Science 601:2792
Haruta M (1997) Catal Today 36:153
Fristrup P, Johansen LB, Christensen CH (2008) Catal Lett 140:184
Jørgensen B, Christiansen SE, Thomsen MLD, Christensen CH (2007) J Catal 251:332
Zheng N, Stucky GD (2007) Chem Commun 37:3862
Enache DI, Knight DW, Hutchings GJ (2005) Catal Lett 103:1
Dimitratos N, Lopez-Sanchez JA, Morgan D, Carley A, Prati L, Hutchings GJ (2007) Catal Today 122:317
Miyamura H, Matsubara R, Miyazaki Y, Kobayashi S (2007) Angew Chem Int Ed 46:4151
The Au/TiO2 catalyst was prepared similarly to the Au/K2Ti6O13 catalyst
Acknowledgments
Center for Sustainable and Green Chemistry is sponsored by the Danish National Research Foundation. Andew DeLaRiva acknowledges support from the US National Science Foundation grant number CTS––0500471, the IREE supplement as well as the PIRE grant OISE-0730277.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klitgaard, S.K., DeLa Riva, A.T., Helveg, S. et al. Aerobic Oxidation of Alcohols over Gold Catalysts: Role of Acid and Base. Catal Lett 126, 213–217 (2008). https://doi.org/10.1007/s10562-008-9688-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9688-x