Abstract
Decellularization is regarded as a xenogenic antigen-reduction technique because it effectively eliminates all cellular and nuclear components while mitigating any negative impact on the composition, biological functionality, and structural integrity of the remaining extracellular matrix. This study aimed to histologically evaluate native, freeze dried and chemically decellularized bovine pericardium membrane. Also, this study focused on preservation of extracellular matrix after decellularization. Bovine pericardium membrane was decellularized by freeze thaw cycle followed by freeze drying and 1% sodium dodecyl sulphate. Unprocessed pericardium was used as control. The effectiveness of Decellularization was assessed based on the reduction of histologically visible nuclei. Decellularization by freeze thaw cycle followed by freeze drying resulted in 17.84% reduction in nuclei content and decellularization by sodium dodecyl sulphate results in 92% reduction in nuclei content compare to control group. Picrosirius red staining for freeze dried group displayed loosely organised, thin collagen bundles that exhibit reddish-yellow birefringence and sodium dodecyl sulfate group revealed dense collagen bundles that are parallelly organised and compact, exhibiting reddish-yellow birefringence and showed good structural integrity. These results suggested that the sodium do decyl sulfate showed optimal decellularization results with better extracellular matrix preservation. It may be a suitable protocol for producing a suitable scaffold for periodontal tissue regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Barrier membranes are extensively employed in the field of dentistry to promote periodontal regeneration. These membranes, when placed over the area of tissue defect, prevent the invasion of cells from the gingival epithelium. Studies have indicated that the protective function needs to be maintained for at least 4–6 weeks for the regeneration of periodontal fibers, and 16–24 weeks for bone augmentation (Hoornaert A et al. 2016). Therefore, barrier membranes should remain in place between the gingiva and alveolar bone for durations exceeding these time frames. This barrier role preserves the area for tissue regrowth and directs specific cells from the periodontal ligament or bone-forming cells toward the damaged region. Bovine pericardium membrane is excellent material as barrier membrane in periodontal regeneration and reconstruction because it demonstrates a strong compatibility with human fibroblasts, promoting their migration, adhesion, and proliferation (Nguyen and Tran 2018). The pericardium membrane exhibits outstanding resistance to tearing in multiple directions, attributed to the presence of fine, wavy, and collagen fibers oriented in various directions (Blatt et al. 2020; Noble et al. 2022). The inherent crosslinking found in the pericardium membrane extends its durability, allowing it to maintain its barrier function for up to 12 weeks after implantation (Radenković M et al. 2021). The utilization of bovine pericardium membrane in clinical practice is a relatively recent development in the field of periodontics, with research on its biological characteristics gaining traction over the past few decades. Biological frameworks taken from animal tissues might be recognized as unfamiliar by the recipient, possibly resulting in an inflammatory reaction or an immune-driven rejection of the tissue. In order to avoid this, cellular antigens must be eliminated from the pericardium while maintaining the integrity of the extracellular matrix. Decellularization is considered a xeno antigen-reduction technique because it reduces the likelihood of degeneration and calcification by eliminating cells and their trash. The objective of the decellularization method is to effectively eliminate all cellular and nuclear components while mitigating any negative impact on the composition, biological functionality, and structural integrity of the remaining extracellular matrix. There is a wide array of decellularization protocols available, including chemical, physical, or enzymatic methods; however, the quest for the most efficient method is still ongoing. Since bovine pericardium must be imported, the overall cost of using it as a barrier membrane in periodontal regenerative therapy rises dramatically, making it difficult to make this material available to the general public. To overcome this hurdle, we at Central Tissue Bank, MAIDS decided to process and prepare the bovine pericardium membrane indigenously. The efficacy of the decellularization process can be monitored histologically by evaluating the nuclear content. The purpose of this study is to determine which method of decellularization is the best and which method will allow us to develop a dependable and cost-effective barrier membrane from bovine pericardial membrane (Fig. 1).
Materials and methods
This study was approved by Institutional Ethical Committee of Maulana Azad Institute of Dental Sciences, New Delhi, India.
Bovine heart collected from a disease-free buffalo obtained from a licensed abattoir immediately after slaughtering was placed in a sterile basin filled with Hank’s Balance Salt Solution and was put into a thermal insulation box at 4ocelcius and transported to the Central Tissue Bank, Maulana Azad Institute of Dental Sciences, New Delhi within one hour. Bovine heart was transferred from transportation box to the sterile tray containing normal saline (Fig. 2a). Under aseptic conditions, the pericardial membrane was dissected from the heart. The pericardial membrane was spread on sterile stainless steel scrubbing tray (Fig. 2b). The external fat was removed from pericardium by using sterile disposable scalpel blade with BP handle and rinsed with Phosphate buffer saline (PBS) to remove blood and body fluids (Fig. 2c). The pericardial membrane was divided into 3 groups.
Processing of bovine pericardium membrane: a bovine heart placed in a sterile tray. b pericardium membrane dissected from heart. c external fat, blood and body fluids removed from the pericardium membrane. d pericardium membrane kept in tris buffer for 48 h in native group. e after thawing pericardium membrane kept in 0.05% sodium hypochlorite in freeze dried group. f pericardium membrane with 1% SDS dissolved in Tris buffer for 48 h at room temperature in SDS group
Group Native: No decellularization: served as control group.
Group freeze dried: Decellularized with Freeze thawing followed by Freeze drying.
Group SDS: Decellularized with 1% Sodium Dodecyl Sulfate (SDS).
Processing of pericardial membrane
In native group Pericardium membrane was kept in Tris buffer (10 mM, pH 7.6) for 48 h at room temperature (Fig. 2d) and then divided into 15 pericardial samples. After 48 h pericardial samples were examined for histological analysis.
In freeze dried group pericardium membrane was transferred from deep freezer into beaker and left to thaw until the ice was not visible/tissue was soft. After thawing pericardium membrane was soaked into 0.05% sodium hypochlorite (Fig. 2e) and shaken in digital laboratory shaker for one and half hour at 190 rpm. Then pericardium membrane was transferred into sterile universal bottle containing sterile purified water and shaken for 5–10 min at 150–200 rpm. This procedure was repeated thrice in different sterile universal bottles with fresh sterile purified water. The pericardium membrane was divided into 15 samples and transferred to sterile glass vials. The vials were subjected to lyophilization in a freeze dryer for 26 h. After 26 h pericardial samples were removed from the Freeze dryer and examined histologically.
In SDS group pericardium membrane was treated with 1% (w/v) SDS dissolved in Tris buffer (10 mM, pH 7.6) for 48 h at room temperature (Fig. 2f). The pericardium membrane was placed in sterile purified water at 4 °C for 12 h. This process was repeated twice. Then pericardium membrane was placed in PBS for three days. PBS solution was changed after every eight hours. The pericardium membrane was divided into 15 samples and examined histologically.
Histological analysis
All 45 Pericardial samples were fixed in 4% buffered formaldehyde for 24 h, processed into paraffin, and then sectioned at 4 μm and stained with hematoxylin & eosin (H&E, Sigma Aldrich, USA) for nuclear material and picrosirius red (PSR, Abcam, USA) for collagen. Three randomly chosen high power fields of the H&E stained sections were photographed at 40 × magnification (Motic BA210 LED, Moticam 3.0 MP) placing a square grid and visible cell nuclei were counted for each slide in each group. The average of 3 high power fields was taken as the final score for each sample in each group. PSR stained sections were then examined under polarized microscope (Nikon Eclipse Ni-U microscope equipped with Nikon Ds-Ri2 digital microscope camera, Nikon Corporation Japan).
Results
Hematoxylin and eosin (H & E) staining
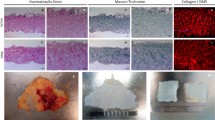
H & E staining of native group revealed a dense tissue structure and pericardial cells were observed within the extracellular matrix. (Fig. 3a). Compared to native bovine pericardium, loose tissue structure with a reduction of cells and nuclei was noted in freeze dried group (Fig. 3b). No obvious cellular content and nuclei was observed in SDS group. However, a dense tissue structure was discernible (Fig. 3c). Histological results indicated a reduction in the cell nuclei present in bovine pericardial tissue after both the decellularization treatments.
For quantitative assessment, the average number of nuclei was calculated for all the three groups from 3 different power fields. The most efficient method of decellularization was determined by the amount of remaining nuclear component (Fig. 4).
Statistical analysis
Kolmogorov–Smirnov and Shapiro–Wilk test was used to check whether the data was following a normal distribution. Data was found to be normally distributed hence parametric test were used for inferential statistics. Analysis of variance (ANOVA) test was used for comparing mean of more than two groups and Post hoc by Bonferroni test was used to identify which group differ from each other and level of statistical significance was set at p value less than 0.05.
Table 1 shows the Mean ± S.D. of Average number of Nuclei of three groups. On comparing the mean difference between the three groups using one way ANOVA, the F value 319.124 and p value of < 0.001 was highly significant.
Multiple comparison for analysing the mean difference of number of nuclei for all 3 groups was performed using the Bonferroni test. The mean difference between Native and freeze dried was 16.067, between native and SDS was 85.267 and between Freeze dried and SDS was 69.200. The mean difference across the 3 groups was highly significant, p < 0.001. This indicates highest number of residual nuclei in the native group followed by freeze drying and SDS group. (Native > Freeze dried > SDS) (Ref. Figures 5 & 6).
Picrosirius red staining (PSR)
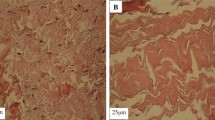
Figure 7a shows PSR staining for native group which revealed collagen bundles were tightly compressed and oriented in a wave-like pattern when viewed under transmitted light. Figure 7b shows PSR staining for freeze dried group which revealed thin collagen bundles which are loosely and parallelly arranged in a wavy pattern. Figure 7c shows thick collagen bundles which are compactly arranged when viewed under transmitted light.
Figure 8a when viewed under polarised microscope, weak birefringence was observed in native group. Figure 8b shows thin collagen bundles which are loosely arranged and show reddish yellow birefringence under polarised microscope. Figure 8c shows thick collagen bundles which are compactly arranged with parallel arrangement and show reddish yellow birefringence under polarised microscope.
Discussion
Decellularization stands out as a highly promising method for creating acellular xenografts. It eliminates key immunogenic cellular elements, including lipid membranes, membrane-linked antigens, and soluble proteins, while preserving the structural integrity of extracellular matrix proteins like collagen and elastin. The cellular elements, such as the nucleic acids found in xenografts, act as potent antigens that stimulate immune responses at the recipient site, leading to the rejection of the graft (Gilbert et al. 2006). Decellularized scaffolds exhibit minimal immunogenicity and are biologically compatible, promoting cell adhesion, growth, and viability. These characteristics make decellularized materials promising for the regeneration of damaged tissues or organs. An efficient decellularization protocol aims to completely eliminate cellular components and nucleic residues, employing a range of methods such as physical, enzymatic, or chemical treatments, either individually or in combination. Decellularization processes induce changes in the structural and mechanical properties of the tissue, but the extent of these alterations varies depending on the specific protocol employed. Zhou et al. noted that decellularization protocols exhibit significant differences in their effects on the histoarchitecture of the extracellular matrix (ECM). (Zhou et al. 2010).
Bovine pericardium (BP) has a long history of clinical applications, particularly in the construction of prosthetic devices for vascular and cardiac surgeries, such as heart valves and repair patches (Athar et al. 2014). BP is made up of a collagen-rich ECM, creating a natural microenvironment that facilitates the migration and proliferation of host cells, thereby fostering tissue regeneration (Gonçalves et al. 2005; Oswal et al. 2007; Li et al. 2011). The current study assesses physical and chemical methods of decellularizing BP, aiming to create a suitable scaffold or barrier membrane for use in periodontal tissue engineering. This study was done at the Central Tissue Bank, Department of Periodontics, M.A.I.D.S., to compare two different methods of decellularization of bovine pericardium with unprocessed pericardium. The membrane was processed with physical (freeze thawing) and chemical (SDS) method of decellularization to evaluate which method between these two decellularized the bovine pericardium effectively.
Samples in freeze dried Group underwent decellularization using a physical method involving a freeze–thaw cycle. This approach to decellularization is known to disrupt cell membranes, release cellular contents, and aid in the removal of cellular materials from the ECM. Rabbani et al. noted that frozen water crystals within cells expand, leading to the bursting of the cell membrane. Freeze-thawing is an effective method for destroying tissue and organ cells. However, any remaining membrane and cellular content can be eliminated through subsequent complementary processes (Rabbani et al.2021). Keane Tj et al. reported that the freeze–thaw cycle results in slight degradation of the ECM structure. This degradation occurs due to the geometric shape of the crystals, which may cause damage to the scaffold. However, this process has minimal effect on the mechanical properties of the ECM (Keane et al. 2015). In our study, we employed a single freeze–thaw cycle to decellularize the bovine pericardium. However, the number of freeze–thaw cycles used in various studies has been variable and often arbitrary. Xing et al. utilized 3 cycles for the decellularization of fibroblast cell sheets (Xing et al. 2015) and Elder et al. used one cycle for lumbar vertebrae cells (Elder et al. 2010).
Martyna Ekiert et al. reported that employing multiple freeze–thaw cycles results in a decrease in the mechanical performance of the material. This process also impacts the internal structure, leading to a significant reduction in the diameters of collagen fibrils, along with alterations in their orientation (Ekiert et al. 2021). Therefore, we performed a single round of freeze thaw cycle to avoid excessive disruption of ECM. Following, the freeze thaw cycle washing with deionized water is an important step for cell removal, because it would facilitate subsequent rinsing and the removal of cellular and nuclear debris from the ECM. After rinsing we performed lyophilization of the samples in a freeze dryer for 26 h. Turner et al., 1981; Quattlebaum et al., 1988 reported that freeze drying reduces the antigenicity of grafts. Meryman et al. (1960) suggested that freeze drying could potentially harm the biochemistry and morphology of cells. Thomas, Edwards, and Damjanovic (1976) reported that freeze-drying might selectively change specific receptors on the cell surface, affecting their functional capacity. This process could also modify the manner in which cell surface antigens are presented to the host (Thomas et al. 1976). Mellonig (1991) noted that freeze-drying influences immune recognition in the host by interfering with the three-dimensional presentation of human leukocyte antigen on the surface of the graft (Mellonig et al. 1991).
Samples in SDS group were decellularized using 1% SDS. Ionic detergents are efficient in solubilizing both cytoplasmic and nuclear membranes (Seddon et al. 2004). The most frequently utilized ionic detergents include sodium dodecyl sulfate (SDS) and sodium deoxycholate. SDS is highly efficient in eliminating cellular components from tissue. In comparison to other detergents, SDS results in the complete removal of nuclear remnants and cytoplasmic proteins, such as vimentin (Woods et al. 2005). Gilbert et al. reported that Sodium deoxycholate is also highly effective in eliminating cellular remnants, but it tends to induce more disruption to the native tissue architecture compared to SDS. There are no documented instances of tissue decellularization using sodium deoxycholate alone, making it challenging to ascertain its specific effect on the remaining ECM of a tissue (Gilbert et al. 2006).
In the present study, samples in SDS group were decellularized with 1% sodium dodecyl sulphate (SDS) for 48 h at room temperature, followed by washing for 3 days. This resulted in complete removal of nuclear and cellular remnants. N Li et al. reported that SDS was effective in solubilizing both the cytoplasmic and the nuclear cellular membranes (Li et al. 2018). Sokol et al. utilized 1% and 0.1% SDS solutions for the decellularization of bovine pericardium. Their study reported that histological examination did not detect any cells in the tissue with either protocol. Furthermore, more than 99% of the nucleic acids were removed from the decellularized bovine matrix (Sokol et al.2020). Nguyen and Tran investigated various SDS concentrations, ranging from 0.05 to 0.3%, for a duration of 12 h. Their study found that 0.15% SDS was adequate to produce an acellular bovine pericardium matrix with a well-preserved architecture, as revealed by H&E staining, in comparison to the native tissue (Nguyen and Tran 2018). Nataliia et al. employed a 0.1% solution of SDS with constant shaking (200 rpm) for a duration of 35 days at 24 °C. Their histological analysis indicated that the decellularized pericardial tissues maintained the extracellular matrix (ECM) components without any cells or nuclei (Shchotkina et al. 2021). Morteza Alizadeh et al. utilized a 1% SDS solution for a duration of 48 h at 40 °C. They conducted vacuum washing to eliminate residual SDS from the pericardium. Their findings suggested that with an increase in the duration and rate of washing, the amount of SDS remaining in the solvent (PBS) decreased. Additionally, the toxicity of SDS in human endothelial cells decreased, leading to increased cell survival (Alizadeh et al. 2019).
The effectiveness of Decellularization was assessed based on the reduction of histologically visible nuclei. In this study, decellularization by freeze thaw cycle results in 17.84% reduction in nuclei content with empty gaps within the ECM compare to native group and decellularization by sodium dodecyl sulphate results in 92% reduction in nuclei content with a uniformly structured ECM. Our results were in accordance with Li et al. who reported that SDS was more effective in removing the cellular and nuclear components. Gilpin et al. found that solely using a freeze–thaw cycle was inadequate for the complete removal of cellular components. This incomplete removal of genetic materials could potentially lead to immunorejection (Gilpin et al. 2017).
On statistical analysis, mean and S.D. of average number of nuclei after decellularization in freeze dried group was 74.53 ± 7.368 which was statistically significant (p < 0.001) compared to native group. Mean and S.D. of average number of nuclei after decellularization in SDS group was 7.22 ± 1.607 which was statistically (p < 0.001) significant compared to native group and freeze dried group. This implies that SDS was efficient in decellularizing the pericardial membrane.
Woods et al. 2005 reported that SDS tends to disrupt the native tissue structure, and causes a decrease in the GAG concentration and a loss of collagen integrity. Yet, there seems to be no indication that SDS eliminates collagen from the tissue. Kasimir et al. also noted that decellularization using the ionic detergent SDS resulted in the complete removal of all cells. However, they observed significant alterations in the matrix structure (Kasimir et al. 2003). The effectiveness of SDS in decellularizing porcine aortic and pulmonary valve leaflets is widely recognized. Histological analysis reveals that after the decellularization process, the major structural components of the valve matrix are preserved (Booth et al. 2002; Kim et al. 2002).
Collagen plays a crucial role in providing tissue strength and structural stability. As such, it is important to find the right balance between removing cells and preserving the extracellular matrix (ECM) when developing an optimal decellularization protocol. Bovine pericardium contains mostly type 1 collagen which is structured into different levels of organisation ranging from fibrils to laminates, fibers and fiber bundles (Allen et al. 1984). In the present study, the effect of decellularization on the structural integrity of the collagen fibres was also observed. To assess the same, picrosirius staining was done on each section of the sample from each group. Picrosirius red is an anionic dye that specifically interacts with the cationic collagen fibrils under low pH conditions. In this interaction, the dye molecules attach to the fibrils with their long axis parallel to that of collagen. This particular arrangement enhances the usual birefringence observed when viewed under a polarized microscope (Junqueira et al.1979). Type I collagen is composed of compactly arranged thick fibrils and consequently presents an intense birefringence of reddish yellow colour.
Our results showed thick collagen bundles compactly and parallelly organised in a crimp pattern seen by picrosirius staining for native Group. However, they showed weak birefringence when examined with a polarised microscope which can be due to change in the structural integrity of the collagen fibrils. Freeze dried Group displayed loosely organised, thin collagen bundles that exhibit reddish-yellow birefringence when viewed under a polarised microscope. The collagen fibrils are parallelly arranged though the structural integrity was compromised. SDS Group revealed dense collagen bundles that are parallelly organised and compact, exhibiting reddish-yellow birefringence when viewed using a polarised microscope and showed good structural integrity. This suggested that structural integrity of collagen fibres was more with SDS decellularized bovine pericardia compare to freeze drying method. Hence, the present study hypothesises the superior nature of the membrane samples treated with SDS. This needs to be validated further with in-vivo animal studies.
Li et al. found that all decellularized bovine pericardia showed preservation of collagen fiber structure when compared to native bovine pericardia. Qi Xing et al. demonstrated that all three decellularization methods—0.5 wt% SDS, 0.05 wt% SDS, and freeze–thaw—largely preserved collagen I and fibronectin. However, they found that the freeze–thaw method better maintained the ultrastructure of the extracellular matrix (ECM), which was contrary to our results.
Conclusion
Within the limits of this study it can be concluded that SDS was more effective in decellularization and preservation of extracellular matrix of bovine pericardial membranes when compared to freeze drying method. However, further in-vitro and animal studies will be required for biocompatibility of bovine pericardium as barrier membrane. Future work will focus on in vivo animal studies.
Data availability
No datasets were generated or analysed during the current study.
References
Alizadeh M, Rezakhani L, Soleimannejad M, Sharifi E, Anjomshoa M, Alizadeh A (2019) Evaluation of vacuum washing in the removal of SDS from decellularized bovine pericardium: method and device description. Heliyon. https://doi.org/10.1016/j.heliyon.2019.e02253
Allen DJ, DiDio LJ, Zacharias A, Fentie I, McGrath AJ, Puig LB, Pomerantzeff PN, Zerbini EJ (1984) Microscopic study of normal parietal pericardium and unimplanted Puig-Zerbini pericardial valvular heterografts. J Thoracic Cardiovasc Surg 87(6):845–855
Athar Y, Zainuddin SL, Berahim Z, Hassan A, Sagheer A, Alam MK (2014) Bovine pericardium membrane and periodontal guided tissue regeneration: a SEM study. Int Med J 21:325–327
Booth C, Korossis SA, Wilcox HE, Watterson KG, Kearney JN, Fisher J, Ingham E (2002) Tissue engineering of cardiac valve prostheses I: development and histological characterization of an acellular porcine scaffold. J. Heart Valve Disease 11(4):457–462
Blatt S, Burkhardt V, Kämmerer PW, Pabst AM, Sagheb K, Heller M, Al-Nawas B, Schiegnitz E (2020) Biofunctionalization of porcine-derived collagen matrices with platelet rich fibrin: influence on angiogenesis in vitro and in vivo. Clin Oral Investig 24:3425–3436. https://doi.org/10.1007/s00784-020-03213-8
Ekiert M, Karbowniczek J, Stachewicz U, Mlyniec A (2021) The effect of multiple freeze-thaw cycles on the viscoelastic properties and microstructure of bovine superficial digital flexor tendon. J Mechan Behavior Biomed Mater 120:104582. https://doi.org/10.1016/j.jmbbm.2021.104582
Elder BD, Kim DH, Athanasiou KA (2010) Developing an articular cartilage decellularization process toward facet joint cartilage replacement. Neurosurgery 66(4):722–727. https://doi.org/10.1227/01.neu.0000367616.49291.9f
Gilpin A, Yang Y (2017) Decellularization strategies for regenerative medicine: from processing techniques to applications. BioMed Res Int. https://doi.org/10.1155/2017/9831534
Gilbert TW, Sellaro TL, Badylak SF (2006) Decellularization of tissues and organs. Biomaterials 27(19):3675–3683. https://doi.org/10.1016/j.biomaterials.2006.02.014
Gonçalves AC, Griffiths LG, Anthony RV, Orton EC (2005) Decellularization of bovine pericardium for tissue-engineering by targeted removal of xenoantigens. J Heart Valve Dis 14(2):212–217
Hoornaert A, d’Arros C, Heymann MF, Layrolle P (2016) Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed Mater 11(4):045012. https://doi.org/10.1088/1748-6041/11/4/045012
Junqueira LC, Bignolas G, Brentani RR (1979) Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochemi J 11:447–455. https://doi.org/10.1007/bf01002772
Kasimir MT, Rieder E, Seebacher G, Silberhumer G, Wolner E, Weigel G et al (2003) Comparison of different decellularization procedures of porcine heart valves. Int J Artif Organs 26:421–427. https://doi.org/10.1177/039139880302600508
Keane TJ, Swinehart IT, Badylak SF (2015) Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 84:25–34. https://doi.org/10.1016/j.ymeth.2015.03.005
Kim WG, Park JK, Lee WY (2002) Tissue-engineered heart valve leaflets: an effective method of obtaining acellularized valve xenografts. Int J Artif Organs 25(8):791–797. https://doi.org/10.1177/039139880202500807
Li N, Li Y, Gong D, Xia C, Liu X, Xu Z (2018) Efficient decellularization for bovine pericardium with extracellular matrix preservation and good biocompatibility. Int Cardiovasc Thoracic Surg 26(5):768–776. https://doi.org/10.1093/icvts/ivx416
Li R, Guo W, Yang B, Guo L, Sheng L, Chen G, Li Y, Zou Q, Xie D, An X, Chen Y (2011) Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 32(20):4525–4538. https://doi.org/10.1016/j.biomaterials.2011.03.008
Mellonig JT (1991) Freeze-dried bone allografts in periodontal reconstructive surgery. Dental Clin N Am 35(3):505–520
Meryman HT (1960) Principles of freeze-drying. Ann New York Acad Sci 85(2):630–640. https://doi.org/10.1111/j.1749-6632.1960.tb49987.x
Nguyen MTN, Tran HLB (2018) Effect of modified bovine pericardium on human gingival fibroblasts in vitro. Cells Tissues Organs 206(6):296–307. https://doi.org/10.1159/000501807
Noble C, Morse D, Lerman A, Young M (2022) Evaluation of pericardial tissues from assorted species as a tissue-engineered heart valve material. Med Biol Eng Comput 60:393–406. https://doi.org/10.1007/s11517-021-02498-5
Oswal D, Korossis S, Mirsadraee S, Wilcox H, Watterson K, Fisher J, Ingham E (2007) Biomechanical characterization of decellularized and cross-linked bovine pericardium. J Heart Valve Disease 16(2):165
Rabbani M, Zakian N, Alimoradi N (2021) Contribution of physical methods in decellularization of animal tissues. J Med Signals Sens 11(1):1–1. https://doi.org/10.4103/jmss.jmss_2_20
Sokol A, Grekov D, Yemets G, Galkin A, Shchotkina N, Dovghaliuk A, Rudenko N, Yemets I (2020) The efficiency of decellularization of bovine pericardium by different concentrations of sodium dodecyl sulfate. Innov Biosyst Bioeng 4:189–198
Quattlebaunr JB, Mellonig JT, Hensel NF (1988) Antigenicity of freeze-dried cortical bone allograft in human periodontal osseous defects. J Periodontol 59(6):394–397. https://doi.org/10.1902/jop.1988.59.6.394
Radenković M, Alkildani S, Stoewe I, Bielenstein J, Sundag B, Bellmann O, Jung O, Najman S, Stojanović S, Barbeck M (2021) comparative in vivo analysis of the integration behavior and immune response of collagen-based dental barrier membranes for guided bone regeneration (GBR). Membranes (basel) 11(9):712. https://doi.org/10.3390/membranes11090712
Seddon AM, Curnow P, Booth PJ (2004) Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta BBA Biomemb. https://doi.org/10.1016/j.bbamem.2004.04.011
Shchotkina NV, Sokol AA, Galkin OY, Yemets GI, Dolinchuk LV, Rudenko NM, Yemets IM (2021) Optimized method of bovine pericardium decellularization for tissue engineering. Wiad Lek 74(4):815–820
Thomas D, Edwards DC, Damjanovic V (1976) The surface properties of spleen cells from CBA/lac mice following freezing and freeze-drying using polyvinylpyrrolidone. Cryobiology 13(2):191–200. https://doi.org/10.1016/0011-2240(76)90132-2
Turner DW, Mellonig JT (1981) Antigenicity of freeze-dried bone allograft in periodontal osseous defects. J Periodontal Res 16(1):89–99. https://doi.org/10.1111/j.1600-0765.1981.tb00952.x
Woods T, Gratzer PF (2005) Effectiveness of three extraction techniques in the development of a decellularized bone–anterior cruciate ligament–bone graft. Biomaterials 26(35):7339–7349. https://doi.org/10.1016/j.biomaterials.2005.05.066
Xing Q, Yates K, Tahtinen M, Shearier E, Qian Z, Zhao F (2015) Decellularization of fibroblast cell sheets for natural extracellular matrix scaffold preparation. Tissue Eng Part C Methods 21(1):77–87. https://doi.org/10.1089/ten.tec.2013.0666
Zhou J, Fritze O, Schleicher M, Wendel HP, Schenke-Layland K, Harasztosi C, Hu S, Stock UA (2010) Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials 31(9):2549–2554. https://doi.org/10.1016/j.biomaterials.2009.11.088
Funding
This study did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
C.G., A.K.L., F.F., and S.T. contributed to the study’s conception and design. Data collection, and analysis was performed by all authors. The first draft of the manuscript was written by C.G., A.D., and S.D. A.K.L., F.F., and S.T. commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they do not have any conflicts of interest.
Ethical approval
This study was approved by Institutional Ethical Committee of Maulana Azad Institute of Dental Sciences, New Delhi, India.
Informed consent
Informed consent was not applicable as its an in vitro study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupt, C., Lamba, A.K., Faraz, F. et al. Histological evaluation of decellularization of freeze dried and chemically treated indigenously prepared bovine pericardium membrane. Cell Tissue Bank 25, 773–784 (2024). https://doi.org/10.1007/s10561-024-10139-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-024-10139-y