Abstract
Stem-cell-based therapies were introduced aiming to overcome the limitations of the existing procedures for regeneration of mineralized tissues. Stem cells isolated from the endometrial tissue and dental pulp have the capacity to differentiate into various functional cells including osteoblasts. However, studies comparing their ability to regenerate mineralized tissue are lacking. The purpose of this study was to compare the proliferation and osteogenic differentiation potential of endometrial stem cells (EnSCs) and dental pulp stem cells (DPSCs) using in vitro cell culture technique. The DPSCs and EnSCs were isolated from human dental pulp and endometrium, respectively. Their proliferation and osteogenic potential were compared in the same osteogenic medium (OM) after 3, 5, 7 and 10 days using the methyl thiazol tetrazolium assay, alizarin red staining, and real-time quantitative reverse transcription polymerase chain reaction (Real-Time qRT-PCR). The EnSCs showed higher proliferation rate compared to DPSCs. Regarding osteogenesis, alizarin red-positive colonies appeared earlier and in greater amounts in DPSCs group. The real-time qRT-PCR demonstrated significantly greater osteogenic potential of DPSCs compared to EnSCs. Our findings revealed significant differences in stem cell properties based on the tissue source. The EnSCs had greater proliferation rate than DPSCs, while DPSCs showed greater osteogenic potential compared to EnSCs in the same OM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The bone tissue is capable of regeneration under many circumstances without scar tissue formation (Nandi et al. 2010). However, bone regeneration in large bone defects is not easily achievable; for this reason, bone transplantation is often used in such cases (Elsalanty and Genecov 2009). Nowadays, tissue engineering offers promising results for the regeneration of injured or lost tissues. Stem cells are one of the three key elements required for tissue engineering (Caplan 2007). Bone regeneration is usually a time-consuming process during which, an acceptably high population of cells must colonize the defect area, proliferate and differentiate into osteoblasts in order to yield efficient productivity and a favorable outcome. These cells may be procured from different sources. Bone marrow stem cells (BMSCs) are a reliable source for this purpose (Wexler et al. 2003) and have been successfully used to enhance the healing of bone defects (Quarto et al. 2001). However, the need for anesthesia prior to procurement is one major obstacle against the use of these cells in the clinical setting. Moreover, these cells lose their differentiation potential by aging and therefore are not a good candidate for use in the elderly (Stenderup et al. 2003). Evidence shows that the differentiation potential of adult mesenchymal stem cells depends on their source of procurement (Alfotawei et al. 2014; Lin et al. 2005; Liu et al. 2007). Thus, for optimal bone tissue engineering, stem cells isolated from different tissues must be compared to find a good source with the ability for use in the clinical setting. Finding such a source of stem cells capable of healing bone defects in the shortest time possible is still a challenge in bone regeneration (Davies et al. 2015). The DPSCs and EnSCs are potential alternatives for use in bone tissue engineering.
Endometrium is a highly dynamic tissue tolerating approximately 400 menstrual cycles during a woman’s reproductive lifetime. This indicates the high cell turnover of this tissue rich of stem cells (Mutlu et al. 2015). Taking a sample from the endometrial lining, compared to bone marrow aspiration, is associated with minimal pain and discomfort for the patient (Gargett et al. 2009). In our previous studies, we showed that EnSCs have odontogenic and osteogenic potential (Tabatabaei et al. 2013a, b). On the other hand, considering the fact that thousands of third molars are extracted and discarded every day, dental pulp can also serve as a suitable source of stem cells for tissue engineering purposes (Li et al. 2011). The osteogenic differentiation potential of DPSCs has been previously confirmed (Kanafi et al. 2014; Tabatabaei et al. 2014).
To date, several studies have compared the differentiation potential of different stem cells and the significant role of stem cell source in the field of tissue engineering is generally accepted (Alge et al. 2010; De Ugarte et al. 2003; Sakaguchi et al. 2005; Yoshimura et al. 2007). Showab et al, in 2007 showed that the population of EnSCs containing mesenchymal stem cells is similar to that of bone marrow and dental pulp (Schwab and Gargett 2007; Shi and Gronthos 2003); however, the osteogenic potential of EnSCs and DPSCs has not yet been compared. In this context we tried to broaden current knowledge of these stem cells by comparing the osteogenic potential of EnSCs and DPSCs.
Materials and methods
Materials
Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS) and antibiotic–antimycotic, trypsin-ethylenediaminetetraacetic acid (EDTA) and Phosphate-buffered saline (PBS) were purchased from Gibco (Paisley, UK). Methyl thiazol tetrazolium (MTT) dye, Alizarine red dye, B-Glycerophosphate, L-Ascorbic acid and dexamethasone were purchased from Sigma-Aldrich (Steinheim, Germany). RNA isolation and cDNA synthesis kits and SYBR®Green PCR mastermix were purchased from Qiagen (Hilden, Germany).
Cell isolation and culture
Endometrial biopsies (n = 3) and extracted teeth (n = 3) were obtained from three independent donors after obtaining informed patient consent according to approval of the ethical committee (IR.SBMU.RIDS.REC.1395.339). Stem cells from each tissue were isolated as described in our previous studies (Tabatabaei et al. 2013a, 2014).
In brief, the procedure was as follows: Primary human dental pulp samples were obtained from unerupted, caries-free third molars of healthy 20–35 year-old adults extracted for clinical reasons after obtaining their informed consent. Tooth surfaces were cleaned and cracked open using a bone cutter to reveal the pulp chamber. The pulp was removed, minced into small pieces and placed in flasks containing DMEM supplemented with 10% FBS and 1% antibiotic–antimycotic (regular medium) in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. The pulp fragments were soaked in culture medium to facilitate their adhesion. The entire medium was changed every 2 days. After 4 weeks, cells from some wells were harvested after exposure to trypsin-EDTA and sub-cultured in complete DMEM until their fourth passage.
Endometrial tissues were collected from ovulating women aged 34–51 years at the Gynecology Department of Shariati Hospital and used with the patients’ informed consent. Patients did not take exogenous hormones for three months before surgery. To isolate EnSCs, endometrial biopsy specimens were placed in Hanks’ Balanced Salt Solution containing antibiotic solution (2%) and the endometrium was scraped off the underlying myometrium. Stem cells were isolated by mincing the tissue, which was incubated with 2 mg/mL type-I collagenase for 2 h at 37 °C. Epithelial cells were separated from the stromal cells by passing the cells through 70 and 45 µm strainers. The cells were then centrifuged at 1000 g for 15 min and purified using Ficoll. Finally, the cells were washed with PBS for several times and cultured in flasks containing DMEM supplemented with 10% FBS, and 1% antibiotic-antimycotic (regular medium), followed by incubation at 37 °C with 5% CO2.
For assays, the fourth passage DPSCs and EnSCs were plated in 48-well culture plates at a concentration of 5 × 103 cells/well. After overnight adhesion of the cells, the medium for two groups of cells was changed to the osteogenic medium (OM: DMEM containing 10−7 M dexamethasone, 10 mM β-glycerophosphate, 50 µg/mL ascorbic acid bi-phosphate, 10% FBS and 1% antibiotic-antimycotic). The control groups contained regular medium (RM: DMEM + 10% FBS + 1% antibiotic-antimycotic). The medium was refreshed twice a week. The cells were incubated in these media for 3, 5, 7 and 10 days. At each time interval, the MTT assay was performed to assess cell viability and proliferation. Alizarin red (AR) staining was performed to assess the formation of calcium nodules (at the mineralization stage) in each culture and the real-time quantitative reverse transcription PCR (Real-Time qRT-PCR) was carried out to assess the mRNA expression level of osteogenesis-related genes in each group.
MTT assay
For the MTT assay, the cells were washed with PBS and the medium in each well was replaced with the medium containing 10% MTT dye (5 mg/mL stock solution). After 3 h of incubation in 37 °C with 10% MTT dye, the medium was removed and the insoluble purple formazan crystals in the viable cells were dissolved using dimethyl sulfoxide (DMSO). Then the optical density (OD) was determined at 570 and 620 nm (reference) wavelength by a microplate reader (Anthos 2020, Salzburg, Austria) and data were expressed as absorbance.
Alizarin red staining
For alizarin red staining, the cells were the cells were washed with PBS and fixed in ice-cold 70% ethanol for 1 h. After several rinsing with deionized water, 2% alizarin red (buffered at a pH of 4 with 0.1% ammonium hydroxide) was added to cells (15 min incubation at room temperature). After several rinsing with deionized water, orange-red calcium nodules were observed using an inverted light microscope (Olympus CK 40, Japan) at 4–10× magnification.
Real-time PCR
For real-time qRT-PCR, total RNA was extracted from induced and control DPSCs and EnSCs at different time intervals, using RNeasy® Plus Mini Kit following the manufacturer’s instructions. After RNA quality and quantity confirmation, reverse transcription (RT) was performed using the QuantiTect® Reverse Transcription kit to obtain complementary DNAs (cDNAs). Quantitative real-time RT-PCR was performed using the LightCycler® 96 System (Roche, Grenzach-Wyhlen, Germany) with the reaction mixture (20 µL) contained 0.5 µL (250 nM final concentration) of forward and reverse primers (Bioneer, Daejeon, South Korea) (Table 1), 2 µL of cDNA, 10 µL of SYBR® Green PCR Master Mix and 7 µL of DNase-free water. Amplification of specific products was performed with the following thermal cycling conditions: initial denaturation at 95 °C for 10 min, denaturation at 95 °C for 10 s, followed by 45 cycles of annealing and extension at 60 °C for 30 s. The threshold was set above the non-template control (NTC) background and within the linear phase of target gene amplification to calculate the cycle number at which the transcript was detected (denoted Ct). Samples were amplified in triplicate and the mean values were calculated. Differences in Ct data were evaluated by the LightCycler® 96 software. Three repetitions were made for each group per each test (mean ± SD). After normalizing the mean CT values (cycle threshold), the changes in expression of ALP (Alkalin phosphatase), OP (Osteopontin) and OC (Osteocalcin) genes were evaluated in comparison with the expression of internal control gene RPL13a (ribosomal protein L13a) using the comparative Ct method (Δ – ΔCt method) with the following equation: ΔCt = Ct (target gene) – Ct (reference gene). The comparative Δ – ΔCt calculation was used to find the difference between the ΔCt of test cells (cultured in OM) and the mean value of the ΔCt of the control cells (cultured in RM). Fold increase in the expression of specific mRNAs was calculated as 2−(Δ–ΔCt). The data were expressed as relative quantification (RQ).
Statistical analysis
All quantitative tests were repeated three times. Statistical analysis was done using GraphPad Prism V.6.01 software (GraphPad, San Diego, CA, USA). Data were expressed as mean ± standard deviation (SD) and analyzed by one-way repeated analysis of variance (ANOVA), followed by Tukey’s post hoc test. Statistical significance was indicated by P < 0.05.
Results
In vitro proliferation of DPSCs and EnSCs
The MTT assay was performed to identify the possible differences in the proliferative activity of DPSCs and EnSCs. At 3 days, EnSCs had equal absorbance in both regular (without differentiation potential) and osteogenic media and the optical density value of EnSCs at this time point was three times that of DPSCs (Fig. 1). The EnSCs continued to proliferate until day 5 and their absorbance was doubled at 5 compared to 3 days. From day 5 on, the absorbance of EnSCs in the regular medium remained constant while the cells in the OM showed a gradual reduction in absorbance until day 10. The DPSCs showed similar absorbance at 3 days in both regular and osteogenic media; thereafter, the proliferation of cells in the regular medium increased until day 10. At 10 days, the absorbance read for DPSCs was not significantly different from that of EnSCs at 5, 7 or 10 days. But, DPSCs present in the OM showed constant absorbance from day 5 until day 10.
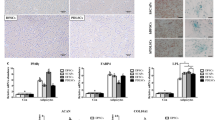
MTT assay. The quantitative comparative analysis the effect of culture time (3, 5, 7 and 10 days after culture) and culture medium [regular medium (RM) and osteogenic medium (OM)] on viability and proliferation of dental pulp stem cells (DPSCs) and endometrial stem cells (EnSCs). Stars in-between the columns indicate significant differences between the groups (P < 0.05)
In vitro osteogenic differentiation of DPSCs and EnSCs
The mineralization assay (qualitative) showed no calcified nodules in the EnSC and DPSC groups (in regular medium; without differentiation potential) during 10 days of culture; whereas matrix calcification was detected at 7 days in the EnSCs/OM and at 5 days in DPSCs/OM group, as indicated by alizarin red S staining (Fig. 2).
In order to confirm the osteogenic differentiation of these matrix-forming cells, real-time RT-PCR (quantitative) was performed. As seen in Fig. 3, ALP activity increased in all group with culture time. At each time point (days 7 and 10 for DPSCs and days 3–10 for EnSCs), the ALP levels were significantly higher in the osteogenic medium than in the regular medium (without differentiation potential) (P < 0.05), implying more active cell differentiation in these groups. EnSCs/OM showed significantly higher ALP activity (P < 0.05) than DPSCs/OM over a three to 7 day observation period. High expression of the OP was detected in DPSCs/OM at day 3, which decreased at 5 and 7 days and significantly increased at 10 days (Fig. 3a). The same profile was also seen for the EnSCs/OM (Fig. 3b); however the increase in OP at three and 10 days was significantly less than that in DPSCs/OM. The OC gene was observed in both DPSC/OM and EnSCs/OM groups at each time point, but the increase of this gene was significantly greater in DPSC/OM group (P < 0.05).
Real-time qRT-PCR. The quantitative comparative analysis the effect of culture time (3, 5, 7 and 10 days after culture) and culture medium [regular medium (RM) and osteogenic medium (OM)] on ALP, OP and OC gene expressions in dental pulp stem cells (DPSCs) and endometrial stem cells (EnSCs). Stars on the columns indicate statistically significant difference (P < 0.05) with the control group (RM, Day 3)
Discussion
The application of tissue engineering is developing fast for regeneration of different tissues. Further advances in this field require a comprehensive knowledge of the comparative capabilities of stem cells isolated from various tissues. Sakaguchi et al. (2005) compared stem cells isolated from different tissues and indicated that some of these cells had higher capability for differentiation into adipose tissue while some others showed greater osteogenic potential. Thus, type of selected cell can significantly affect the success of treatment. To date, several studies have compared the differentiation potential of different stem cells (Alge et al. 2010; De Ugarte et al. 2003; Sakaguchi et al. 2005; Yoshimura et al. 2007). However, the osteogenic potential of EnSCs and DPSCs has not yet been compared. Therefore, the current study compared the proliferation rate and osteogenic potential of these two groups of cells.
The proliferation of DPSCs has been compared previously with that of BMSCs and it has been shown that the cell population and proliferative activity of DPSCs are greater than those of BMSCs (Alge et al. 2010). However, our study compared DPSCs and EnSCs and showed higher proliferation rate of EnSCs. Previous studies have also indicated very high proliferation rate of EnSCs (Schwab et al. 2005). In our study, DPSCs displayed a significantly lower optical density 3 days after the culture, which resulted in lower yield of DPSCs in cultures even after 10 days. Notably, this effect may be due to the isolation methods used for different mesenchymal cell sources potentially contributing to differences in early cell growth (Davies et al. 2015). This result may also indicate that EnSCs potentially require a shorter in vitro culture period to reach cell numbers sufficient for clinical application. In general, proliferation has an inverse relationship with differentiation and increased differentiation is associated with decreased cell proliferation (Bilezikian et al. 2008). Gradual reduction in absorbance of EnSCs/OM from day 5 on indicates the gradual entry of these cells into the differentiation phase. However, in DPSCs/OM group, after proliferation until day 5, a gradual stability of cell population was noted. This could be explained by the fact that differentiation in these group of cells is well progressed before leaving the proliferative cycle.
The current study showed that DPSCs had a significantly higher osteogenic potential than EnSCs. Following alizarin red staining, calcified nodules in DPSCs/OM were observed on day 5; while these nodules in EnSCs/OM were seen on day 7. Relative expression levels of some osteogenic-related genes (ALP, OP, and OC) were quantitatively detected by Real-Time RT-PCR, and the results showed that expression of OP and OC was significantly higher in DPSC/OM compared to EnSCs/OM (Fig. 3). It has been demonstrated that differentiation of stem cells progresses through three general phases: proliferation, extracellular matrix maturation, and mineralization, with characteristic changes in gene expression in each stage. ALP is an early marker of osteogenic differentiation, which expresses during the period of matrix deposition and maturation. Osteopontin (OP) peaks twice during the end of the proliferative period and then again later during the period of mineralization; while osteocalcin (OC) may be considered a marker for terminal differentiation expressed concomitantly with mineralization (Bilezikian et al. 2008). In our study, comparison of the osteogenic differentiation of DPSCs and EnSCs showed that culture of DPSCs in OM result in higher expression of OC and OP at 10 days compared to the EnSCs. However, the expression of ALP in the first days was higher in EnSCs/OM compared to DPSCs/OM, which may be due to the higher population of the former cells in the medium. Alge et al. (2010) compared DPSCs and BMSCs and showed higher proliferation and activity of ALP in DPSCs compared to BMSCs. Ponnaiyan and Jegadeesan (2014) also reported that the proliferation rate and differentiation potential to osteogenic lineage were greater in DPSCs compared to the BMSCs. In contrast, Takahashi et al. compared the odontogenic differentiation potential of DPSCs and dental follicle cells and reported higher activity of dental follicle cells in terms of expression of odontogenic genes (Takahashi et al. 2004). Hakki et al. (2015) compared DPSCs and periodontal ligament stem cells and showed that although DPSCs had higher proliferation potential, the two groups of cells were not significantly different in terms of expression of mineralized tissue-associated genes. One limitation of our study was that the donors of EnSCs and DPSCs were not matched. This limitation, according to some researchers (Alge et al. 2010), may affect the results.
In cell-based therapy, cells should be preferred according to their potentials (proliferation, and differentiation) and the targeted tissue. EnSCs and DPSCs cells are fibroblast-like, plastic adherent mesenchymal stem cells (MSC), and they have ability to differentiate into various specialized cells. Co-expression of two perivascular cell markers, CD146 and platelet-derived growth factor-receptor β (PDGF-Rβ) suggests that these cells are similar to MSC from bone marrow and dental pulp (Shi and Gronthos 2003; Schwab and Gargett 2007). In spite of many similarities between DPSCs and EnSCs, these populations in the current study demonstrated remarkable differences with respect to their proliferation and mineralization.
Conclusion
According to our results, both MSCs (EnSCs and DPSCs) demonstrated osteogenic differentiation. Although the EnSCs exhibited higher proliferation potential, the DPSCs showed more remarkable osteogenic competence. This may have general implications for selection of candidate cells for bone engineering. Future studies could compare stability and differentiation of these stem cells under in vivo conditions.
References
Alfotawei R, Naudi KB, Lappin D, Barbenel J, Di Silvio L, Hunter K, McMahon J, Ayoub A (2014) The use of TriCalcium Phosphate (TCP) and stem cells for the regeneration of osteoperiosteal critical-size mandibular bony defects, an in vitro and preclinical study. J Craniomaxillofac Surg 42:863–869. doi:10.1016/j.jcms.2013.12.006
Alge DL, Zhou D, Adams LL, Wyss BK, Shadday MD, Woods EJ, Gabriel Chu TM, Goebel WS (2010) Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med 4:73–81. doi:10.1002/term.220
Bilezikian JP, Raisz LG, Martin TJ (2008) Principles of bone biology: two-volume set. Academic Press, Cambridge
Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213:341–347. doi:10.1002/jcp.21200
Davies OG, Cooper PR, Shelton RM, Smith AJ, Scheven BA (2015) A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J Bone Miner Metab 33(4):371–382. doi:10.1007/s00774-014-0601-y
De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH (2003) Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 174(3):101–109. doi:10.1159/000071150
Elsalanty ME, Genecov DG (2009) Bone grafts in craniofacial surgery. Craniomaxillofac Trauma Reconstr 2(3):125–134. doi:10.1055/s-0029-1215875
Gargett CE, Schwab KE, Zillwood RM, Nguyen HPT, Wu D (2009) Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod 80(6):1136–1145. doi:10.1095/biolreprod.108.075226
Hakki SS, Kayis SA, Hakki EE, Bozkurt SB, Duruksu G, Unal ZS, Turaç G, Karaoz E (2015) Comparison of mesenchymal stem cells isolated from pulp and periodontal ligament. J Periodontol 86(2):283–291. doi:10.1902/jop.2014.140257
Kanafi M, Ramesh A, Gupta P, Bhonde R (2014) Dental pulp stem cells immobilized in alginate microspheres for applications in bone tissue engineering. Int Endod J 47(7):687–697. doi:10.1111/iej.12205
Li J-H, Liu D-Y, Zhang F-M, Wang F, Zhang W-K, Zhang Z-T (2011) Human dental pulp stem cell is a promising autologous seed cell for bone tissue engineering. Chin Medical J (Engl) 124(23):4022–4028
Lin Y, Luo E, Chen X, Liu L, Qiao J, Yan Z, Li Z, Tang W, Zheng X, Tian W (2005) Molecular and cellular characterization during chondrogenic differentiation of adipose tissue-derived stromal cells in vitro and cartilage formation in vivo. J Cell Mol Med 9(4):929–939. doi:10.1111/j.1582-4934.2005.tb00389.x
Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B (2007) Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 25(3):750–760. doi:10.1634/stemcells.2006-0394
Mutlu L, Hufnagel D, Taylor HS (2015) The endometrium as a source of mesenchymal stem cells for regenerative medicine. Biol Reprod 92(6):138. doi:10.1095/biolreprod.114.126771
Nandi S, Roy S, Mukherjee P, Kundu B, De D, Basu D (2010) Orthopaedic applications of bone graft & graft substitutes: a review. Indian J Med Res 132:15–30
Ponnaiyan D, Jegadeesan V (2014) Comparison of phenotype and differentiation marker gene expression profiles in human dental pulp and bone marrow mesenchymal stem cells. Eur J Dent 8(3):307–313. doi:10.4103/1305-7456.137631
Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M (2001) Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 344(5):385–386. doi:10.1056/NEJM200102013440516
Sakaguchi Y, Sekiya I, Yagishita K, Muneta T (2005) Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 52(8):2521–2529. doi:10.1002/art.21212
Schwab K, Gargett C (2007) Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Human Reprod 22(11):2903–2911. doi:10.1093/humrep/dem265
Schwab KE, Chan RWS, Gargett CE (2005) Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril 84:1124–1130. doi:10.1016/j.fertnstert.2005.02.056
Shi S, Gronthos S (2003) Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 18(4):696–704. doi:10.1359/jbmr.2003.18.4.696
Stenderup K, Justesen J, Clausen C, Kassem M (2003) Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33(6):919–926. doi:10.1016/j.bone.2003.07.005
Tabatabaei FS, Ai J, Kashi TSJ, Khazaei M, Kajbafzadeh A-M, Ghanbari Z (2013a) Effect of dentine matrix proteins on human endometrial adult stem-like cells: in vitro regeneration of odontoblasts cells. Arch Oral Biol 58(7):871–879. doi:10.1016/j.archoralbio.2013.01.013
Tabatabaei FS, Dastjerdi MV, Jazayeri M, Haghighipour N, Dastjerdie EV, Bordbar M (2013b) Comparison of osteogenic medium and uniaxial strain on differentiation of endometrial stem cells. Dent Res J (Isfahan) 10(2):190–196
Tabatabaei F, Jazayeri M, Ghahari P, Haghighipour N (2014) Effects of equiaxial strain on the differentiation of dental pulp stem cells without using biochemical reagents. Mol Cell Biomech 11(3):209–220
Takahashi M, Yamada Y, Ozawa R, Ohya M, Ito K, Ueda M (2004) Expression of odontoblastic-related genes in human dental follicle cells, dental pulp stem cells, and oral mucosal cells. Int J Oral-Med Sci 3(1):41–48. doi:10.5466/ijoms.3.41
Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM (2003) Adult bone marrow is a rich source of human mesenchymal ‘stem’cells but umbilical cord and mobilized adult blood are not. Br J Haematol 121(2):368–374
Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I (2007) Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 327(3):449–462. doi:10.1007/s00441-006-0308-z
Acknowledgements
This study was financially supported by the School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No. 9009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tabatabaei, F.S., Torshabi, M. In vitro proliferation and osteogenic differentiation of endometrial stem cells and dental pulp stem cells. Cell Tissue Bank 18, 239–247 (2017). https://doi.org/10.1007/s10561-017-9620-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-017-9620-y