Abstract
Osteochondral defects may progress to osteoarthritis. Many attempts have been developed to overcome this issue, including osteochondral autografts and allografts. The goal of this study was to develop a new protocol for storage of human osteochondral allografts. Osteochondral plugs were randomly allocated in the following groups: control, immediate freezing up to −70 °C, cooling at 4 °C, and storage at 37 °C. Samples from the cooling at 4 °C and storage at 37 °C groups were stored in tubes containing medium plus human albumin and analyzed after 1, 3, and 14 days. The frozen groups’ samples were cryopreserved for 1 year in cryotubes containing medium only (FM), medium plus human albumin (FA), and medium plus human albumin and glucose (FG) and were then analyzed. Analysis involved histological study with hematoxylin-eosin and Safranin O and a modified Live/Dead assay. In samples stored both at 37 and 4 °C, analysis showed statistically significant higher cellular mortality at 14 days compared to 1 and 3 days, but mortality in the 4 °C group was lower. In the freezing protocols, the FA group showed less cellular mortality than the FM and FG groups. Cooling at 4 °C offers better preservation capacity than storage at 37 °C, but both offer the capacity for preservation for 14 days. Adding human albumin to the storage medium is useful in reducing cellular mortality in samples frozen for 1 year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteochondral defects progress to osteoarthritis, causing pain and disability (Huntley et al. 2005). Many attempts have been made to treat cartilage defects and prevent joint degeneration. Surgical options include microfracture, autologous chondrocyte implantation, and osteochondral autografts or allografts. However, none of these options have been proven to completely restore the structure and function of articular cartilage (Safran et al. 2008).
Osteochondral autografts have the advantage of transplanting viable metabolically active chondrocytes, but show limited donor availability and donor-site morbidity (Fritz et al. 2008). Osteochondral allografts, supplied as fresh, cold-stored, or frozen tissue, have been described to present good results in many reviews (Bobic et al. 1999; Lonner et al. 1999; Hangody et al. 2008; Swan et al. 2006; Feeley et al. 2009; Reddy et al. 2009; Lattermann et al. 2009). Fresh osteochondral allografts have success rates around 75 % at 5 years, but need to be implanted within 7 days after harvest in order to achieve maximum chondrocyte viability in the implants (Pearsall et al. 2004). Recently, the use of fresh osteochondral allografts has been reported to show survival rates of 91, 84, 69 and 59 % at 10, 15, 20, and 25 years, respectively (Raz et al. 2014). Frozen grafts offer the possibility of long-term banking, which allows screening for infectious diseases (Pallante et al. 2009). However, crystals generated from ice disrupt the extracellular matrix and play a major role in chondrocyte death during cryopreservation (Muldrew et al. 2000; Pegg et al. 2006; Pegg 2010; Abazari et al. 2013). Despite the damage caused to both chondrocytes and matrix, it is possible to obtain 50 % cell survival in isolated cells and 30 % in articular tissue by using controlled freezing (Schachar and Mcgann 1986).
Cryoprotective agents help maintain chondrocytes viability by permeating isolated cells relatively quickly and diffusing slowly through multicellular tissue systems (Carsi et al. 2004). Its exposure may be prolonged in order to achieve adequate concentration in the center of the tissue, while taking care to avoid the toxic effects in the cells near the surface (Carsi et al. 2004). Glycerol and dimethyl sulphoxide (DMSO) are the most used cryoprotective agents in tissue banking. Recent findings, using magnetic resonance imaging analysis, showed that DMSO offered a potential protective effect over chondrocytes when compared to phosphate buffered saline (Laouar et al. 2007). In addition, glycerol and DMSO have similar diffusion coefficients, but cartilage was more permeable to glycerol (Carsi et al. 2004).
Osteochondral allografts stored in lactated Ringer’s solution could be implanted within 7 days after harvest, but storage could be extended to approximately 2 weeks if the allografts were kept in culture media (Dulbecco´s Modified Eagle´s Medium) in order to improve chondrocyte viability (Ball et al. 2004). However, another study showed no differences in viability index between the replaced medium and non-replaced groups of osteochondral allografts stored at 4 °C for 60 days (Csonge et al. 2002). Osteochondral allograft storage in fetal bovine serum seems superior to serum-free culture medium (Pennock et al. 2006).
The goal of this study was to propose a new protocol for storage of osteochondral fragments. Different mediums and temperatures able to maintain cellular viability and the extracellular matrix’s overall structure for future transplantation were used to for osteochondral fragments storage.
Methods
Population study
Two hundred and twenty cylindrical osteochondral allografts were collected from twenty-two patients, aged 55–70 years old, who were undergoing total knee replacement at the National Institute of Traumatology and Orthopedics, Rio de Janeiro, Brazil, between January 2011 and January 2012. Osteochondral plugs were cut from specimens of the distal femur, the proximal tibia, and the patella articular surfaces that had been removed and discarded by the surgeon from patients during total knee replacement. Mosaicplasty tools were used to achieve the 0.6 cm diameter cylindrical harvests. Specimens were selected for harvesting from cartilage with macroscopically normal appearance and that was least 2 mm thick (Fig. 1a). Each patient provided ten cylindrical osteochondral fragments, which were transported in saline solution at 4 °C to the laboratory and randomized in one of the following groups: (a) control, (b) storage at 37 °C(S), (c) cooling at 4 °C(C), or (d) freezing at −70 °C(F) (Table 1).
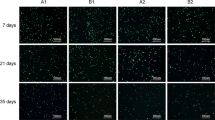
Osteochondral allografts processing and cellular death viability assay a Cylindrical osteochondral fragment (6 mm). Cartilage was minced and digested in 2.5 % collagenase. Then DAPI and ethidium bromide were added to complete Live/Dead assay. b Dead cells identified by ethidium bromide staining (red). c All cells identified by DAPI staining (blue). (Color figure online)
In the control group (C0), osteochondral plugs were processed just after collection to evaluate the tissue’s cell population. In the storage condition at 37 °C, osteochondral plugs were kept in Iscove’s medium (Gibco, USA) supplied with human albumin (Sigma-Aldrich, Switzerland) for 1 day (S1), 3 days (S3), and 14 days (S14). In the 4 °C storage condition, osteochondral fragments were conditioned in the same mediums and stored for 1 day (C1), 3 days, (C3) and 14 days (C14). Samples from S and C groups were stored in polypropylene tubes containing 40 mL of media for every ten fragments, which was changed every 3 days.
In the group frozen at −70 °C, osteochondral plugs were dipped into three different solutions: Iscove’s medium plus DMSO 10 % (Sigma-Aldrich, Brazil) (FM), Iscove’s medium (Gibco, USA) supplied with human albumin plus DMSO 10 % (FA), and Iscove’s medium (Gibco, USA) plus Glucose 10 % plus DMSO 10 % (FG). For freezing, we used Mr. Frosty by Nalgene ® to lower the temperature to −70 °C at a rate of −1 °C/min. Samples were stored for 1 year. Samples of this group were stored individually in cryotubes containing 2 mL of solution for each fragment. In this third protocol, we analyzed the effects of cryopreservation media on cell death. For thawing, samples were kept at 37 °C for 3 min in an incubator and then processed.
Samples from patients with a prior history of infection, surgery, or corticosteroids infiltration in the affected knee were not included in the study. This research study was approved by the Institutional Board (0040.0.305.000-07 SISNEP/CONEP) and informed consent was obtained from all donors.
Histological analysis
Cartilage was fixed in 10 % buffered formalin and further processed for paraffin embedding. Tissue sections of 5 µm were stained with hematoxylin-eosin for general tissue structure evaluation and Safranine O to detect proteoglycans distribution. Two independent observers evaluated the structural changes in the tissue samples using the Mankin Modified Score grading system (Rohde et al. 2004).
Chondrocytes isolation
For analysis, the cartilage was separated from bone in each plug. Selected fragments were minced, transferred to a sterile tube, and incubated at 37 °C for 18 h in cell culture dishes containing 0.5 % collagenase. After incubation, the remnants were homogenized using scissors and a Pasteur pipette. Cell suspension was transferred to a 50 mL tube containing Iscove’s Medium supplemented with 10 % Bovine Fetal Serum. After centrifugation at 1,500 rpm at 4 °C, the supernatant was discarded and the pellet was resuspended. A Neubauer Chamber was used for cell counting. A minimum of 2 × 104 cells per sample was necessary for measurement.
Cell viability assay
The viability assay was performed on each sample after storage of the cartilage fragments. Cell viability was analyzed using a modified LIVE/DEAD® assay (LIVE/DEAD® Viability/Cytotoxicity Kit for mammalian cells, Invitrogen—Brazil). To concentrate the chondrocytes for analysis, a cytocentrifuge (Cientec, CT2000—New Química) was used. The total number of cells was first identified by DAPI’s blue emission (Invitrogen). Then, ethidium bromide was added, and the cells were incubated for 30 min at 37 °C in an incubator to stain damaged or dead cells in red (Fig. 1b, c). After staining, cells were rinsed twice in PBS in order to remove excess dye. For each group, a quantitative analysis was performed using a fluorescent microscope (Eclipse E600, Nikon). Photomicrographs were taken in order to have at least 2,000 cells per group. Images were merged and analyzed using Adobe Photoshop and Image J (Adobe). Cellular death index was obtained by assessing the proportion of dead and total cell count.
Statistical analysis
All data were analyzed by GraphPad Prism for Windows 5 considering the ratio between dead cells and the total cell number. Results were presented as mean and corresponding standard deviation. Statistical analysis was performed according to Student’s t test. Results were considered significant when p was <0.05.
Results
For the samples stored at 37 °C, we observed significantly higher cellular mortality on the 14th day (14.1 ± 11.9 %) when compared to the 1st (4.2 ± 2.8 %) and to the 3rd day (5.7 ± 3.7 %). For samples cooled at 4 °C, cellular mortality was significantly higher in the 14th day (7.1 ± 3.3 %) when compared to the 1st (2.6 ± 5 %) and to the 3rd day (5.3 ± 2.6 %). Cellular mortality was significantly higher in the samples stored at 37 °C on the day 14 when compared to the samples cooled at 4 °C on day 14. (14.1 ± 11.9 vs. 7.15 ± 3.3 %, respectively) (Table 2).
Samples stored at −70 °C showed significantly higher cellular mortality than control no matter which medium the samples were stored in (Table 3). However, samples stored in the FA group presented significantly lower cellular mortality than samples stored in the FG group (20.6 ± 3.9 vs. 28 ± 7.4 %).
To determine whether the cartilage structure was affected, Mankin criteria were used for evaluation. Overall cartilage structure showed no hypocellularity or any significant changes in superficial structures (data not shown). All samples stored, independent of the method used, did not show matrix degeneration in the cartilage by evidence of hematoxylin and eosin (Fig. 2a) or Safranin O stain (Fig. 2b).
Discussion
Osteochondral allografts have been used for treatment of articular cartilage defects due to their high rate of functional outcomes (Bobic et al. 1999; Lonner et al. 1999; Swan et al. 2006; Hangody et al. 2008; Feeley et al. 2009; Lattermann et al. 2009; Reddy et al. 2009). However, their use has been threatened by the fear of infectious disease transmission to the host. Therefore, allografts must be properly stored for screening until approved for implantation. Many protocols have been developed in the past few years, but today there is still no consensus on a protocol for routine use.
Two factors are imperative to define storage success: chondrocyte viability and matrix integrity (Laouar et al. 2007). Hence, the effective preservation procedure for cartilage, aimed for allograft transplantation, seems to depend on both the storage time and medium used to achieve these goals.
We propose, for short storage time, to plunge the cartilage in human albumin diluted in Iscove’s medium at 4 °C for up to 14 days. Our results have shown that cartilage storage at 4 °C maintained longevity and viability.
These results reflect other previous studies involving samples stored at 4 °C with different storage times and mediums. Pennock et al. (2006) showed that osteochondral allograft storage in medium plus fetal bovine serum was superior to serum-free culture media after 28 days of storage. Ball et al. (2004) showed that osteochondral allografts stored in lactated Ringer’s solution allowed implanting within 7 days after harvest, but storage duration could be extended to approximately 2 weeks if the allografts were kept in culture medium. Teng et al. (2008) showed that adding recombinant IGF-1 and an apoptosis inhibitor called ZVAD-fmk to DMEM improved chondrocyte survival in bovine osteochondral grafts. Onuma et al. (2012) observed that supplementation of University of Wisconsin solution with allogeneic serum enhanced the viability of rat osteochondral samples for 14 days. Pearsall et al. (2004) had also indicated that osteochondral allografts could be refrigerated in a culture medium in temperatures ranging from 2 to 8 °C maintaining 67 % of chondrocyte viability for up to 44 days prior to implantation.
When comparing samples stored at 4 and 37 °C, we found statistically significant difference only at day 14 with higher mortality in storage at 37 °C. In contrast, Pallante et al. (2009) showed that cell viability was higher in goat osteochondral allografts stored at 37 °C for 2–6 weeks instead of at 4 °C. More recently, storage of canine osteochondral allografts for 28 days at 37 °C in serum-free media showed better results than at 4 °C (Garrity et al. 2012).
Our results showed that, for long term storage at −70 °C, a protein-rich medium is necessary, such as Iscove´s medium supplemented with human albumin and diluted in a cryoprotective agent, such as DMSO. Judas et al. found that using a nutrient mixture by itself would allow the recovery of a greater amount of chondrocytes. DMSO increased chondrocyte viability when compared to glycerol or no cryoprotective agent (Judas et al. 2007). Acosta et al. (2007) suggested that improving freezing conditions might moderate the impact of cryopreservation on chondrocyte viability and extracellular matrix degradation. Recently, a study showed that multiple cryoprotective agent solutions are less cytotoxic than single ones (Almansoori et al. 2012).
Ohlendorf et al. (1996) criticized the use of DMSO as a cryoprotective agent in a study using calf samples, as chondrocyte survival seemed to be limited to the superficial layer only, although DMSO increased the overall number of surviving cells. In our study, which used DMSO as a single cryoprotective agent, histological analysis showed that tissue structure was maintained through all layers.
Our results also suggest that cell viability decreases over storage time in protocols involving both cooling at 4 °C and storage at 37 °C. These data are similar to findings of previous research studies involving human (Pearsall et al. 2004; Pallante et al. 2009) and sheep (Willians et al. 2004) allografts stored in Ringer Lactate and Dulbeco medium, respectively.
One limitation of our study was that we used samples from patients with osteoarthritis. We believe that this limitation was minimized by the fact that all sample groups received samples from the same patient profile. Studies using osteoarthritic cartilage samples for evaluating the effect of cryopreservation showed that the percentage of viable cells depends on the condition of chondrocytes before cryopreservation (Rendal-Vazquez 2001; Xia 2008). Therefore, the results obtained are comparable due to the protocols used. However, this age-related effect on chondrocyte viability cannot be overruled. We are also aware that this patient donor profile is not adequate for allograft transplantation because of the same reasons.
Another limitation was the short follow up time for non-frozen samples, since there are already other studies showing longer periods of observation in similar storage temperatures. In this study, we wanted to compare in the same study protocols using human allograft storage at both 37 and 4 °C. Our next steps involve continuing the experiments for a longer time using healthy cadaver cartilage donors for the protocols that showed lower cell mortality indexes.
We opted to use samples sized 6 mm in order for the samples to fit in cryotubes, which could favor storage and reduce unnecessary wasting of fragments not used for transplant in the case of massive storage of samples. Fening et al. studied the effect of storage medium tonicity on osteochondral plug diameter. This study affirmed that the greater the diameter of the plug, the more difficult the graft insertion would be, increasing the contact force during implantation and probably raising cellular death at the surface (Fening et al. 2011). Reddy et al. (2009) also prefer the 6 mm diameter plug because it better fits the defect. However, other studies involving fresh samples used larger diameter fragments, such as 8 mm (Pallante et al. 2012) and even 15 mm (Ball et al. 2004). We believe the diameter chosen in this study was adequate for our purpose.
In conclusion, our findings allow us to propose that, in a protein-rich medium, either cooling at 4 °C or freezing at −70 °C are acceptable for the preservation of osteochondral allografts for up to 14 days or 1 year, respectively.
References
Abazari A, Jomha NM, Elliott JAW, Mc Gann LE (2013) Cryopreservation of articular cartilage. Cyobiology 66:201–209
Acosta CA, Izal I, Ripalda P, Forriol F (2007) Cell viability and protein composition in cryopreserved cartilage. Clin Orthop Relat Res 460:234–239
Almansoori KA, Prasad V, Forbes JF, Law JK, McGann LE, Elliott JAW, Jomha NM (2012) Cryoprotective agent toxicity interactions in human articular chondrocytes. Cryobiology 64:185–191
Ball ST, Amiel D, Williams SK, Tontz W, Chen AC, Sah RL, Bugbee WD (2004) The effects of storage on fresh human osteochondral allografts. Clin Orthop Relat Res 418:246–252
Bobic V (1999) Osteochondral autologous graft transplantation in the treatment of focal articular lesions. Semin Arthroplasty 10(1):21–29
Carsi B, Lopez-Lacomba JL, Sanz J, Marco F, Lopez-Duran L (2004) Cryoprotectant permeation through human articular cartilage. Osteoarthr Cartil 12:787–792
Csonge L, Bravo D, Newman-Gage H, Rigley T, Conrad EU, Bakay A, Md Strong, Pellet S (2002) Banking of osteochondral allografts, Part II. Preservation of chondrocyte viability during long term storage. Cell Tissue Bank 3:161–168
Feeley BT, Rodeo SA (2009) Osteochondral allograft transplantation. Tech Knee Surg 8(1):22–28
Fening SD, Mihnovets J, Jones MH, Midura RJ, Miniaci A (2011) The effect of storage medium tonicity on osteochondral allografts plug diameter. Arthroscopy 27(2):188–193
Fritz J, Janssen P, Gaissmaier C, Schewe B, Weise K (2008) Articular cartilage defects in the knee—basics, therapies and results. Injury 39:S50–S57
Garrity JT, Stoker AM, Sims HJ, Cook JL (2012) Improved osteochondral allograft preservation using serum-free media at body temperature. Am J Sports Med 40(11):2542–2548
Hangody L, Vásárhelyi G, Hangody LR, Sükösd Z, Tibay G, Bartha L, Bodó G (2008) Autologous osteochondral grafting—technique and long-term results. Injury 39(S1):S32–S39
Huntley JS, Bush PG, Mcbirnie JM, Simpson AH, Hall AC (2005) Chondrocyte death associated with human femoral osteochondral harvest as performed for mosaicplasty. J Bone Joint Surg Am 87(2):351–360
Judas F, Rose S, Teixeira L, Lopes C, Mendes AF (2007) Chondrocyte viability in fresh and frozen large human osteochondral allografts: effect of cryoprotective agent. Transpl Proc 39:2531–2534
Laouar L, Fishbein K, Mcgann LE (2007) Cryopreservation of porcine articular cartilage: MRI and biochemicals results after different freezing protocols. Cryobiology 54:36–43
Lattermann C, Romine SE (2009) Osteochondral allografts: state of the art. Clin Sports Med 28:285–301
Lonner JH, Cohen MW (1999) Fresh osteochondral allografts for focal articular defects of the knee. Semin Arthroplasty 10(1):15–20
Muldrew K, Novak K, Yang H, Zernicke R, Schachar NS, McGann LE (2000) Cryobiology of articular cartilage: ice morphology and recovery of chondrocytes. Cryobiology 40:102–109
Ohlendorf C, Tomford WW, Mankin HJ (1996) Chondrocyte survival in cryopreserved osteochondral articular cartilage. J Orthop Res 14:413–416
Onuma K, Urabe K, Naruse K, Uchida K, Itoman M (2012) Allogenic serum improves cold preservation of osteochondral allografts. Clin Orthop Relat Res 470(10):2905–2914
Pallante AL, Bae WC, Chen AC (2009) Chondrocyte viability is higher after prolonged storage at 37 °C than at 4 °C for osteochondral grafts. Am J Sports Med 37(Suppl. 1):24S–32S
Pallante AL, Görtz S, Chen AC, Healey RM, Chase DC, Ball ST, Amiel D, Sah SL, Bugbee WD (2012) Treatment of articular cartilage defects in the goat with frozen versus fresh osteochondral allografts: effects on cartilage stiffness, zonal composition, and structure at six months. J Bone Joint Surg 94-A(21):1984–1995
Pearsall AW, Tucker JA, Hester RB, Heitman RJ (2004) Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med 32(1):125–131
Pegg DE (2010) The relevance of ice crystal formation for the cryopreservation of tissues and organs. Cryobiology 60(3 suppl):S36–S44
Pegg DE, Wang L, Vaughan D, Hunt CJ (2006) Cryopreservation of articular cartilage. Part 2: mechanisms of cryoinjury. Cryobiology 52:347–359
Pennock AT, Wagner F, Cm Roberson, Harwood FL, Bugbee WD, Amiel D (2006) Prolonged storage of osteochondral allografts. J Knee Surg 19(4):265–272
Raz G, Safir OA, Backstein DJ, Lee PTH, Gross AE (2014) Distal femoral fresh osteochondral allografts: follow-up at a mean of twenty-two years. J Bone Joint Surg Am 96(13):1101–1107
Reddy S, Benjamin C, Feeley BT (2009) Autologous osteochondral grafting of articular lesions in the knee. Tech Knee Surg 8(1):14–21
Rendal-Vázquez ME, Maneiro-Pampín E, Rodríguez-Cabarcos M, Fernández-Mallo O, Ullibarri IL, Andíon-Nuñez C, Blanco FJ (2001) Effect of cryopreservation on human articular chondrocyte viability, proliferation, and collagen expression. Cryobiology 41:2–10
Rohde RS, Studer RK, Chu CR (2004) Mini-pig fresh osteochondral allografts deteriorate after 1 week of cold storage. Clin Orthop Relat Res 10(427):226–233
Safran MR, Kim H, Zaffagnini S (2008) The use of scaffolds in the management of articular injury. J Am Acad Orthop Surg 16(6):306–311
Schachar NS, Mcgann LE (1986) Investigations of low-temperature storage of articular cartilage for transplantation. Clin Orthop Relat Res 208:146–150
Swan KG, Rabalais RD, Mccarty E (2006) Osteochondral autograft plug transfer for cartilage lesions. Tech Knee Surg 5(3):149–157
Teng MS, Yuen AS, Kim HT (2008) Enhancing osteochondral allograft viability. Clin Orthop Relat Res 466(8):1804–1809
Williams RJ, Dreese JC, Chen C (2004) Chondrocyte survival and material properties of hypothermically stored cartilage. Am J Sports Med 32(1):132–139
Xia Z, Murray D, Hulley Triffit JT, Price AJ (2008) The viability and proliferation of human chondrocytes following cryopreservation. J Bone J Surg 90(9):1245–1248
Acknowledgments
The authors would like to thank Ana Luiza Brito de Almeida for helping with the histological analysis and Nayara Gomes for helping with the statistical analysis. The authors gratefully acknowledge funding support from FAPERJ.
Conflict of interest
The authors declare no conflict of interest regarding this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Sousa, E.B., Aguiar, D.P., Barcelos, J.F.M. et al. Approaches to preserve human osteochondral allografts. Cell Tissue Bank 16, 425–431 (2015). https://doi.org/10.1007/s10561-014-9486-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-014-9486-1