Abstract
Skin allografts represent an important therapeutic resource in the treatment of severe skin loss. The risk associated with application of processed tissues in humans is very low, however, human material always carries the risk of disease transmission. To minimise the risk of contamination of grafts, processing is carried out in clean rooms where air quality is monitored. Procedures and quality control tests are performed to standardise the production process and to guarantee the final product for human use. Since we only validate and distribute aseptic tissues, we conducted a study to determine what type of quality controls for skin processing are the most suitable for detecting processing errors and intercurrent contamination, and for faithfully mapping the process without unduly increasing production costs. Two different methods for quality control were statistically compared using the Fisher exact test. On the basis of the current study we selected our quality control procedure based on pre- and post-processing tissue controls, operator and environmental controls. Evaluation of the predictability of our control methods showed that tissue control was the most reliable method of revealing microbial contamination of grafts. We obtained 100 % sensitivity by doubling tissue controls, while maintaining high specificity (77 %).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skin allografts are an important therapeutic choice in the management of burns and skin loss of various origins (Kearney 2005). They have ideal properties as biological dressings and play a major role in the surgical management of extensive wounds when autologous tissue is not available. The increasing use of allograft skin to treat patients with extensive burns, trauma and soft tissue injuries has led to a sharp increase in the number of skin banks in the last 20 years (Kagan et al. 2005). Despite massive recent developments in bioengineered products, skin allografts are still the gold standard for treating burns and severe skin loss of various origins and are widely used in burn units and surgery departments.
The literature contains reports of sporadic cases of bacterial infections due to bacterial contamination of skin allografts (Monafo et al. 1976), but although contaminated skin grafts are occasionally transplanted, clinically significant infections caused by the allografts rarely occur (Eastlund 2006). To ensure tissue graft microbiological safety, skin banks perform microbiological testing of skin prior to processing with antibiotic solutions (Csonge et al. 1995) and after processing, on finished product samples. As a precautional measure, donor skin contaminated with virulent bacteria and critical pathogens, such as Clostridium spp, is not processed but discarded (Pianigiani et al. 2010). Sources of tissue contamination include infected donors, procurement and processing environments and materials or media used during processing. It is therefore essential for graft safety that procedures to minimise the risk of contamination be used in all stages of processing.
In Europe, skin banks are governed by European directives (Directive 2004/23/EC, Directive 2006/17/EC, Directive 2006/86/EC) that suggest the application of Good Manufacturing Practices (GMP) (EU Guidelines GMP 2008). In Italy, pharmaceutical laboratories and cell factories rigorously apply GMP and must be certified, but skin banks only have guidelines inspired by GMP that do not completely regulate the sector. These guidelines are subject to interpretation and personalisation in the management of microbiological quality control and in the identification of indicators for microbiological control of tissue (Vicentino et al. 2009). In the absence of a univocal indication of the degree and type of controls to carry out to monitor levels of contamination, it is important that critical phases of processing be subject to microbiological and particle count. In Italy skin banks process skin in grade A laminar flow cabinets in a GMP grade B environment, according to national regulations (EU Guidelines GMP 2008; Linee Guida CNT 2007).
In order to demonstrate that tissue processing occurs aseptically and to ensure that the environment or operators are not sources of contamination, it is necessary to conduct other quality controls besides destructive microbiological testing of the processed tissues for fast and slow growing aerobic and anaerobic bacteria and fungi on tissue by 21-day skin cultures (Pianigiani et al. 2010; Pirnay et al. 2012). This period enables detection of slow-growing microorganisms, which would not be identified by 3- or 7-day skin cultures. In our experience, about 1.2 % of 935 donors samples were contaminated by pathogenic slow-growing fungi. If no bacteria, yeasts or fungi grow in 21 days the sample is declared negative. In our skin bank, only aseptic tissues can be validated and distributed; as tissues do not undergo final sterilisation but are decontaminated with either antibiotic solutions or glycerol, it is of the utmost importance to demonstrate aseptical handling and processing.
Controls also include microbiological analysis of processing media, microbiological and particle counts in the laboratory environment and microbiological control of operators. It is also important to validate critical processes to ensure that the results obtained coincide with those expected and that the processes themselves are standardised and reproducible.
Such an elevated number of tests is onerous and complex for non-profit organisations, which is the case of skin banks in Italy. We therefore conducted a study to determine what type of quality controls for skin processing are the most suitable for detecting processing errors and in-process contamination, and for faithfully mapping the process without unduly increasing production costs.
Materials and methods
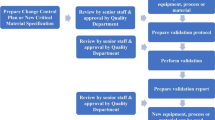
The aim of the present paper was to identify a highly sensitive and specific protocol for microbiological controls in the processing of skin for human use (Fig. 1).
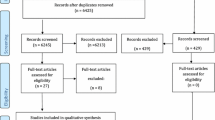
The study (study A) was conducted on an initial group of 206 donors processed at the Siena Skin Bank 104 (50.5 %) heart beating donors; 98 (47.6 %) non-heart beating donors; 4 (1.9 %) living donors], 52 (25.2 %) female and 154 (74.8 %) male, ranging in age from 14 to 74 years (mean 55.4 years). Tissue from 176 donors was cryopreserved (107 skin, 69 de-epidermised dermis—DED) and tissue from 30 donors was glycerol-preserved (Fig. 2). The study was based on analysis of the following quality control procedures:
Study A was conducted on 206 donors divided into two groups on the basis of different quality control protocols (QC1 and QC2). QC1 protocol was based on three environmental controls (air sampler, contact plate and settle plates) and five operator controls (glove print—both hands, wrists and upper-chest). QC2 protocol was based on three environmental controls (air sampler, contact plate and settle plates) and two operator controls (glove prints—both hands) and post-processing media control. Phase 1 Results of quality controls (QC1 and QC2). Phase 2 Results of tissue tests
-
1.
Environmental microbiological controls by SAS-PBI air sampler (aspiration 1000 L/h), 90 mm diameter settle plates for the duration of packaging and 55 mm diam. contact plates at the end of processing;
-
2.
Operator control by 55 mm plates in contact with fingers of both hands for 1 s. (glove print);
-
3.
Additional controls to determine what additional tests are needed for routine tissue quality control, in which the 206 donors were divided into two groups of 103 donors on the basis of two different Quality Control protocols (QC1 and QC2):
-
a.
in QC1 additional quality controls consisted in microbiological testing of the wrists and upper chest of the operator;
-
b.
in QC2 additional quality controls consisted in microbiological testing of the final processing medium.
-
a.
-
4.
Tissue control (post-processing) by packaging a tissue fragment of at least 20 cm2 in a bag similar to those used for donor tissue and sending it to be tested for aerobic and anaerobic bacteria, and fast-, moderate- and slow-growing fungi. This test was used to confirm the reliability of quality controls.
On the basis of the results of study A, we conducted a second study (study B) on 107 donors with double microbiological testing of a processed tissue sample. This group of donors consisted of 43 (40.2 %) multi-organ donors, 62 (57.9 %) multi-tissue donors and 2 (1.9 %) living donors [37 (34.6 %) female, 70 (65.4 %) male, age range 18–74 years, mean age 54.7 years)]. The method of conservation was cryopreservation in 83 (77.6 %) and glycerol preservation in 24 cases (22.4 %). Thirty-four/107 randomly selected donors were subject to further microbiological control (Fig. 3).
Statistical comparison between the above-described two groups of donors was performed by using the Fisher exact test with a significance level of 95 %. Sensitivity (SE) and specificity (SP) were also estimated. In a health decision-method, sensitivity/specificity represents the capacity of the method to correctly identify an adverse/favourable health event in the subset of all adverse/favourable events, and it is expressed with the following formula:
where TP, TN, FP and FN are the number of true positives (adverse events correctly identified as adverse), true negatives (favourable events correctly identified as favourable), false positives (favourable events wrongly identified as adverse) and false negatives (adverse events wrongly identified as favourable), respectively.
Results
Out of a total of 206 donors, 49 (23.8 %) tissues were found to be contaminated after processing and 157 (76.2 %) were negative. The two groups of donors were analysed considering the results of operator, environmental and additional controls in a first phase and taking the results of the tissue test after processing as confirmation in a second phase (Fig. 2—Study A).
Comparing the data of two groups in Study A (QC1 and QC2 which used different methods of environmental and operator control), we found no statistically significant differences. Specificity was quite high in both groups (89.2 and 90.4 %, respectively) whereas sensitivity was low (24.1 and 25.0 %, respectively), indicating that the method successfully recognised negative tissue with negative environmental and operator test results but had difficulty in recognising positive tissue with negative environmental and operator test results.
On the basis of poor sensitivity, we therefore doubled tissue controls, eliminating operator controls and the additional tests not envisaged by the regulations (EU Guidelines GMP 2008; Linee Guida CNT 2007). This was calculated to enable economic savings without compromising safety. Since we wanted to increase the sensitivity of our tissue controls, we investigated whether increasing post-processing controls on tissue would confirm negative results, considering that the distribution of microbial contamination on tissue is not homogeneous.
Doubling the controls on tissue of 107 donors, we found negativity in both tests for 91 donors (group B1) and positivity in one test in the other 16 donors (group B2). We then conducted a further control on 23 donors of group B1, which was negative for all samples, and on 11 donors of group B2, which was negative in seven samples and positive in four (Fig. 3—Study B).
The method based on double control of tissue had good sensitivity (100 %) and was sufficiently specific (77.0 %). This means that it identified all true positives, namely all tissues with positive microbiological test results (sensitivity 100 %). It also identified true negatives with low error, because some positives were false. From our point of view, high accuracy in identifying microbiological contamination is preferable because it ensures tissue safety. However, we aimed to improve the specificity of the method in order to reduce the need for reprocessing or rejection of tissues due to false positives.
Discussion
The Siena Skin Bank, established in 2000, processes skin from 110–130 donors per year (about 400,000 cm2/year). More than 2,500,000 cm2 of homologous skin has been used for the treatment of 10,817 patients with burns and other types of skin loss. To the best of our knowledge, in the 12-year period of our activity, there has been only one severe adverse reaction in a patient due to post-grafting anaphylactic reaction to vancomycin used in skin processing.
The skin bank processes tissue of heart-beating (51.2 % on average) and non-heart-beating donors (48.8 % on average). In multi-organ donors, skin is harvested after aortic clamping (Vuola and Pipping 2002). In non-heart-beating donors skin can be procured up to 24 h after death if the body is cooled or refrigerated within 12 h of death.
The skin is procured under aseptic conditions after appropriate cleaning and disinfection to reduce resident microbial flora that lives primarily in and around hair follicles. Adequate shaving of the donor areas is therefore necessary to minimise skin contamination, particularly when retrieval is carried out in the morgue (Mathur et al. 2009). In order to reduce the degree of microbial contamination, the donor is cleaned with povidone-iodine solution, rinsed with sterile saline and disinfected with tincture of chlorhexidine (Pianigiani et al. 2010; May et al. 1985).
A discard rate of 4.9 % due to microbiological contamination is reported in the literature (Obeng et al. 2001), whereas in our skin bank, specific antibiotics, determined by antibiogram, enable us to reduce the discard rate from 8.5 % (no corrective action) to 1.2 %, which is an excellent result. In fact, cryopreserved grafts contaminated by commensals can be re-processed in glycerol (Mackie 1997) in order to save tissue from being discarded. Specimens contaminated by critical pathogens, such as Clostridium, are discarded prior to processing procedures.
Because of the rising demand for viable tissue and the threat posed by increasingly resistant bacteria, we only validate aseptic tissue in order to avoid severe adverse reactions due to microbial contamination. We do not even accept a low bioburden of commensals, such as Propionibacterium acnes, as its pathogenic potential and virulence could cause infections in severe burn patients (Perry and Lambert 2011). Skin allografts can therefore be accepted for clinical use when the bacteriological and mycological cultures and all quality controls are negative.
To ensure the quality of processed tissue, the Italian legislation on tissue banks (Linee Guida CNT 2007) states: “If tissues or cells are processed while exposed to the environment, without a subsequent microbial inactivation process, air quality with particle counts and microbial colony counts equivalent to those of grade A of the European Guide to GMP (EU Guidelines GMP 2008), is necessary, and a background environment appropriate for processing the tissue or cell concerned:
-
For corneas or amniotic membrane used as cornea a background environment of at least grade D is required;
-
For skin and tissues used inside the body (e.g. unsterilised vessels, valves, muscle), the background environment must be at least grade B at rest.”
European Commission Directive 2006/86/EC does not make distinctions between different types of tissues, requiring grade A processing and grade D background.
Obviously, to review microbiological environmental and quality control testing, several controls have to be performed: microbiological environmental monitoring, container integrity testing, pre-sterilization bioburden testing (for terminally sterilised tissues), media fill medium growth promotion testing and sterility testing. Indeed, it is important to bear in mind that for GMP processing of tissue, it is not enough to have negative quality control results for tissue. Processing must occur in the best aseptic conditions and these conditions must be standardised, monitored and constantly maintained. Sterility testing is a quality control test used as part of product release for products required to be sterile. It has significant statistical limitations, and can only detect gross contamination. Final sterility testing may even be unreliable, especially when antibiotics remain on tissues (Eastlund 2006). This is why it is indispensable to process tissues in clean rooms, where rigorous limits on particle and microbiological contamination are observed. Processing procedure should be validated (e.g. by media fill testing) in order to ensure that no further contamination is added to skin samples during manipulation. Clean room operators should also be regularly validated to demonstrate that they do not contaminate gowns during gowning up.
As sustained by various authors (Eastlund 2006) it should be recalled that microbiological controls on tissue can give false negatives due to antibiotic residues and that further apparently redundant controls are necessary to identify false negatives. Tissue should also be appropriately placed in washing solution that removes processing antibiotics before grafting, and the procedure must be validated.
The distribution of microorganisms in tissues is not homogeneous, although for practical purposes we consider it to be so (Vicentino et al. 2009; Pirnay et al. 2012). Thus, the more controls a tissue undergoes (by destructive testing of final products), the greater the probability of identifying even minor contamination (few CFUs).
On the basis of the results of our study we can therefore state that transmission of bacteria by skin allografts is possible, but infrequent. One case of infectious disease from fresh skin allograft has been reported (Monafo et al. 1976; CNT, WHO, SOHO V&S project 2011). Our bank does not supply fresh tissue, as we do not consider it to be microbiologically safe, because the brief interval between procurement and transplant is insufficient for microbiological testing. In fact, we have to consider that, although skin is placed on the external surface of the body, it is greatly used for severely burned patients who have no skin barrier and develop immunosuppression by various mechanisms, being at higher risk of death from overwhelming infections.
The double-control post-processing strategy proved to maintain a sensitivity of 100 % and high specificity (77 %). Further studies are needed to increase the specificity of our methods, although our primary objective (high sensitivity, i.e. high accuracy in identifying microbiological contamination, ensuring tissue safety) has already been achieved.
References
CNT, WHO, SOHO V&S project (2011) Working group 2—other tissues. In: Notify. Exploring vigilance notification for organs, tissues and cells. A global consultation. Compositori Ed. Bologna pp 38–39
Commission Directive 2004/23/EC of the European Parliament and Council on quality and safety standards for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells
Commission Directive 2006/17/EC implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells
Commission Directive 2006/86/EC implementing Directive 2004/23/EC of the European Parliament and of the Council as regards traceability requirements, notification of serious adverse reactions and events and certain technical requirements for the coding, processing, preservation, storage and distribution of human tissues and cells
Csonge L, Pellet S, Szenes A, István J (1995) Antibiotics in the preservation of allograft and xenograft skin. Burns 21:102–105
Eastlund T (2006) Bacterial infection transmitted by human tissue allograft transplantation. Cell Tissue Banking 7:147–166
EU guidelines to good manufacturing practice medicinal products for human and veterinary use—Annex 1, Ed. 2008
Kagan RJ, Robb EC, Plessinger RT (2005) Human skin banking. Clin Lab Med 25:587–605
Kearney JN (2005) Guidelines on processing and clinical use of skin allografts. Clin Dermatol 23:357–364
Linee guida per il prelievo, la processazione e la distribuzione di tessuti a scopo di trapianto—Centro Nazionale Trapianti ed. 2007
Mackie DP (1997) The Euro Skin Bank: development and application of glycerol-preserved allografts. J Burn Care Rehabil 18:S7–S9
Mathur M, De A, Gore M (2009) Microbiological assessment of cadaver skin grafts received in a skin bank. Burns 35:104–106
May SR, Wainwringht JF, DeClement FA (1985) Variables determining the amount of microbial contamination on cadaveric allograft skin used as a biological wound dressing. Burns 11:242–251
Monafo WW, Tandon SN, Bradley RE, Condict C (1976) Bacterial contamination of skin used as a biological dressing. A potential hazard. JAMA 235:1248–1249
Obeng MK, McCauley RL, Barnett JR, Heggers JP, Sheridan K, Schutzler SS (2001) Cadaveric allograft discards as a result of positive skin cultures. Burns 27:267–271
Perry A, Lambert P (2011) Propionibacterium acnes: infection beyond the skin. Expert Rev Anti Infect Ther 9:1149–1156
Pianigiani E, Ierardi F, Cuciti C, Brignali S, Oggioni M, Fimiani M (2010) Processing efficacy in relation to microbial contamination of skin allografts from 723 donors. Burns 36:347–351
Pirnay JP, Verween G, Pascual B, Verbeken G, De Corte P, Rose T et al (2012) Evaluation of a microbiological screening and acceptance procedure for cryopreserved skin allografts based on 14 day cultures. Cell Tissue Bank 13:287–295
Vicentino M, Rodriguez G, Saldias M, Alvarez I (2009) Guidelines to implement quality management systems in microbiology laboratories for tissue banking. Transplant Proc 41:3481–3484
Vuola J, Pipping D (2002) Maintaining a glycerolized skin bank—a practical approach. Burns 28:S31–S33
Acknowledgments
We would like to acknowledge Dr. Gabriele Cevenini (Department of Biomedical Engineering, University of Siena) for statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pianigiani, E., Ierardi, F. & Fimiani, M. Importance of good manufacturing practices in microbiological monitoring in processing human tissues for transplant. Cell Tissue Bank 14, 601–607 (2013). https://doi.org/10.1007/s10561-012-9356-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-012-9356-7