Abstract
Although allografts for anterior cruciate ligament (ACL) replacement have shown advantages compared to autografts, their use is limited due to the risk of disease transmission and the limitations of available sterilization methods. Gamma sterilization has shown detrimental effects on graft properties at the high doses required for sufficient pathogen inactivation. In our previous in vitro study on human patellar tendon allografts, Electron beam (Ebeam) irradiation showed less detrimental effects compared to gamma sterilization (Hoburg et al. in Am J Sports Med 38(6):1134–1140, 2010). To investigate the biological healing and restoration of the mechanical properties of a 34 kGy Ebeam treated tendon allograft twenty-four sheep underwent ACL replacement with either a 34 kGy Ebeam treated allograft or a non-sterilized fresh frozen allograft. Biomechanical testing of stiffness, ultimate failure load and AP-laxity as well as histological analysis to investigate cell, vessel and myofibroblast-density were performed after 6 and 12 weeks. Native sheep ACL and hamstring tendons (HAT, each n = 9) served as controls. The results of a previous study analyzing the remodeling of fresh frozen allografts (n = 12) and autografts (Auto, n = 18) with the same study design were also included in the analysis. Statistics were performed using Mann–Whitney U test followed by Bonferroni-Holm correction. Results showed significantly decreased biomechanical properties during the early remodeling period in Ebeam treated grafts and this was accompanied with an increased remodeling activity. There was no recovery of biomechanical function from 6 to 12 weeks in this group in contrast to the results observed in fresh frozen allografts and autografts. Therefore, high dose Ebeam irradiation investigated in this paper cannot be recommended for soft tissue allograft sterilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allograft use has increased significantly in recent years. In 2005, members of the American Orthopaedic Society for Sports Medicine used more than 60,000 allografts in sports medicine surgical procedures (McAllister et al. 2007; Cohen and Sekiya 2007).
The main advantages of using allografts for ligament augmentation and replacement are lack of donor site morbidity, the possibility to treat patients with multiple ligament injuries and those requiring revision surgery, reduced operation times, reduced postoperative pain and a lower risk of arthrofibrosis (Harner et al. 1996; Cohen and Sekiya 2007).
However, there is a risk of donor to recipient disease transmission when non-sterilized allografts are used. In 2002 the U.S. Center for Disease Control reported 26 cases of bacterial infection associated with allografts from one donor, which resulted in the death of one patient (Kainer et al. 2004; Balsly et al. 2008). Furthermore, there is a risk of virus transmission (Nemzek et al. 1994; Simonds et al. 1992; Buck et al. 1989). The combination of donor screening, serological testing, bioburden reduction methods (such as washing with antibiotics and/or surfactants) and terminal “in-package” low dose (10–15 kGy) gamma sterilization have been shown in the US to reduce, but not eliminate pathogen transmission (Kainer et al. 2004; Simonds et al. 1992).
As a consequence the laws in some countries, including Germany, require musculoskeletal allograft transplants to be sterilized with a validated method. Chemical sterilization methods such as peracetic acid treatment have gained wide acceptance. Peracteic acid sterilization has proven to be effective (Scheffler et al. 2007) and ideal for the sterilization of tissue transplants including bone, dermis, amnion, fascia lata, tendons and ligaments. But, although chemical sterilization methods had been proven to provide a high level of tissue safety, there have been reports describing some drawbacks of sterilized tendon allograft including inflammatory reactions and delayed remodeling (Jackson et al. 1990; Roberts et al. 1991; Scheffler et al. 2008a). Gamma irradiation is perhaps the most widely applied allograft transplant sterilization method. It has been shown that a dose of more than 30 kGy is required to achieve sufficient pathogen inactivation (Fideler et al. 1994; Pruss et al. 2002). However, many studies have shown dose dependent effects with doses above 20 kGy significantly altering the biomechanical properties of soft tissue grafts (Gibbons et al. 1991; Rappe et al. 2007; Salehpour et al. 1995; McGilvray et al. 2010). Current clinical studies showed significantly higher graft failure rates, following gamma irradiation with 25 kGy (Krych et al. 2008; Sun et al. 2009; Rappe et al. 2007). There remain concerns in some disciplines about the suitability of such sterilized allografts for use in the most demanding clinical applications where sufficient mechanical strength and appropriate remodeling behaviour are of the utmost importance. There is therefore strong interest in improving sterilization methods for soft tissue allografts.
Electron beam (Ebeam) irradiation has been used for the sterilization of medical devices and in radiation therapy (Gerbi et al. 2009) and is of particular interest since it can be operated as a fast throughput method with the possibility to more accurately control the sterilization environment (Reid 1998) and the applied dosage than is possible with conventional gamma sterilization facilities. The effectiveness is comparable to gamma radiation (Seto et al. 2008).
In vitro studies from our group investigating dose-dependent effects of irradiation on tissue transplants showed that in contrast to those treated with gamma irradiation, tendons irradiated with Ebeam at doses below 30 kGy showed no significant reduction in stiffness and failure load when compared with untreated tendons (Hoburg et al. 2010).
While the effects of gamma irradiation on allografts have been well documented, few studies exist examining the effect of Ebeam irradiation on soft tissue allograft. Furthermore, previous studies only analyzed the biomechanical properties of Ebeam irradiated tissues prior to transplantation. To our knowledge, no study has been published analysing the in vivo performance of Ebeam irradiated grafts during early remodeling.
The aim of this study was to determine the influence of Ebeam irradiation (34 kGy) on early remodeling and biomechanical properties of free tendon allografts used for ACL reconstruction in an established in vivo animal model (Hunt et al. 2005; Allen et al. 1998; Radford et al. 1996; Seitz et al. 1997).
We hypothesized that Ebeam irradiation of soft-tissue grafts will not affect early remodeling and will allow recovery of biomechanical strength to that of non-sterilized grafts 12 weeks after transplantation.
Materials and methods
The M. flexor digitalis superficialis, a model for hamstring tendons in humans, was harvested under aseptic conditions from female, 2.5 year-old sheep, placed in sterile bags and stored at −80°C. Six grafts were used as fresh frozen allografts (control) and eighteen grafts were irradiated with 34 kGy Ebeam.
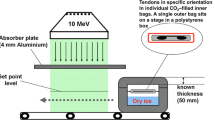
Before the irradiation, bags were rinsed with CO2 assuring an oxygen content less than 0.1% in the bag. After this packaging, the grafts were transported on dry ice to the irradiation facility GSE 80 of Gamma Product Services GmbH, Radeberg, Germany to be irradiated with 34 kGy Ebeam maintaining a temperature of approximately −78°C. Afterwards grafts were stored for at least 10 days at −20°C before being used for ACL reconstruction.
Eighteen 2.5 year-old female Merino mix sheep (mean weight 72.7 kg) underwent ACL replacement surgery using the 34 kGy Ebeam irradiated free tendon allograft. Six additional sheep received a fresh frozen allograft. Contra-lateral knees and native hamstring tendons served also as controls. The data from previously published experiments with fresh frozen non-sterilized allo- and autografts from an identical sheep model (Scheffler et al. 2008b) were included in the analysis of results.
All animal procedures were conducted according to the guidelines of the National Institute of Health for the use of laboratory animals. All animals were checked for bony maturity by dental status. Normal health status was confirmed by a veterinarian.
Histological analysis and mechanical testing were performed after 6 and 12 weeks.
Surgical preparation
On the day of surgery, the grafts were thawed at room temperature and soaked in sterile isotonic NaCl until use. Graft length was between 60 and 70 mm. Each end of the graft was augmented with No. 2 Ethibond Excel polyester sutures in a baseball stitch technique as previously described (Scheffler et al. 2008b).
Before the operation, animals received 3.0 g Unacid (Ampicillin and Sulbactam) intravenously as antimicrobial prophylaxis. The left hind limb of each animal was shaved and prepared with a standard sterile technique.
Anesthesia was initiated with intravenous injection of 20 mg/kg thiopental-natrium and 5 ml of 0.5 mg fentanyl. It was continued with endotracheal administration of isofluran and nitric oxide throughout the surgical treatment. Every 30 min an intravenous application of 5 ml 0.5 mg fentanyl was administered to maintain analgesia. At the end of operation, a fentanyl patch was attached to the left foreleg. Furthermore, animals received 1.5 ml finadyne 50 mg/ml i.m. for the first 3 days after surgery for pain relief.
Surgical procedure
In each animal the flexor tendon was harvested from the left hind limb under the same conditions as in our previous study and stored aseptically at −20°C (Scheffler et al. 2008b).
An open ACL reconstruction was performed on each left hind limb as previously described (Scheffler et al. 2008b; Weiler et al. 2002). All surgeries were performed by the same orthopaedic surgeon. The joint was opened by a medial arthrotomy. The patella and the Hoffa fat pad were retracted laterally and the ACL was excised. In deep flexion, a guide pin was introduced into the femoral foot print of the ACL and overdrilled at a length of 20 mm matching the diameter of the prepared graft. The graft was pulled into the tunnel and fixed at the femoral cortex with a fixation button. The tibial tunnel was placed in an identical fashion into the tibial footprint of the ACL and drilled through the tibial cortex. The graft was fixed by placing multiple knots of the augmented graft sutures onto a bone bridge. Before final fixation, the knee was moved through several cycles of full flexion and extension to eliminate slack of the graft sutures and to pretension the graft. Afterwards, a sequential closure of the retinaculum, the soft tissue layers and skin was conducted. Postoperative X-rays were done to confirm the placement of the bone tunnels and the graft.
Postoperative treatment
After the operation full weight bearing was allowed in all animals. They were housed in an indoor facility and separated into groups of 2 for the first 3 days. Body temperature, wound status and gait pattern were controlled daily. After 2 weeks, stitches were removed and all animals were released to an outdoor facility without any restriction of motion.
After 6 and 12 weeks after ACL replacement, half of the animals in both treatment groups were sacrificed with an overdose of thiopental sodium and potassium chloride. Before the left knee joint was removed, the range of motion and swellings or other noticeable pathological findings were reported. Skin and all soft tissue structures around the joint were left intact. During biomechanical testing, each knee was inspected intraarticulary for inflammation, effusion, the synovial coverage of the ACL graft, the status of the cartilage and any degenerative changes.
Biomechanical testing
Biomechanical testing was performed as described previously (Scheffler et al. 2008b).
Briefly, the femur and tibia were potted in polymethylmethacrylate and mounted on aluminium clamps for fixation in the mechanical testing machine (model 1455; Zwick; Ulm; Germany). Two loading conditions were simulated: an anterior-posterior (AP) drawer test of the femur-tendon-tibia complex and a load to failure test of the femur-tendon-tibia construct. AP drawer test was done in 60° of flexion. After preloading the knee joint with 5 N, an AP load of ±50 N was applied perpendicular to the longitudinal axis of the tibia for 10 times at a speed of 120 mm/min. AP laxity was recorded from the tenth cycle for each specimen.
After excision of the posterior cruciate ligament, load to failure test was performed at 30° of flexion. The longitudinal axis of the ACL allograft was aligned parallel to the loading direction of the testing apparatus. After applying a preload of 5 N to the femur-tendon-tibia complex, the load to failure test was performed at a cross-head speed of 120 mm/min. The failure mode and stiffness (in the linear region between 30 and 90% of the maximum load) were analyzed using in-house software. Native femur-ligament-tibia complexes from the non-operated leg served as controls.
Histological analysis
After biomechanical testing, the allograft was removed and cross- and longitudinal sections of the intact midsubstance of the graft were prepared. These were fixed in formalin 4% for 2–3 days, automatically dehydrated for 3 days and embedded in Paraffin. 4 μm thick serial cuts were prepared and mounted on slides with 3% silane (Sigma Chemical, St. Louis, MO).
For the visualization and histological evaluation, a high resolution microscope (Leica DMRB, Leica GmbH, Bensheim, Germany) linked to a digital image analysis system (KS 400 Imaging System release 3.0.; Carl Zeiss Vision, Eching, Germany) was used. Samples were analyzed with 50× and 100× magnification for overview and identification of the regions of interest and 200× magnification for semiquantitative statistical analysis and cell shape evaluation.
For cell density evaluation and descriptive analysis haematoxylin-eosin (HE) staining was performed. Quantification of total cell number was conducted on longitudinal sections, where 10 defined regions of interest (each 0.364 mm²) were analyzed and cell number/mm² was assessed. For descriptive analysis, cell distribution pattern, cell morphology, inflammatory reactions and the alignment of the collagen fibrils were assessed.
To evaluate vascular and myofibroblast density, established protocols were used (Unterhauser 2004) with few modifications. For vessel detection, cross sections were immunostained with rabbit antihuman von Willebrandt factor (Factor VIII) -antibody which binds on the endothelial surface of blood vessels and enables one to detect both large vessels and small capillaries and to analyze revascularization from an early capillary phase. The tissue samples were hydrated and pretreated with 0.1% pronase for 10 min at 37°C. Ten percent normal horse serum (Vector Laboratories Inc., Burlington, CA, USA) was applied for 20 min at room temperature to block non-specific binding sites. The antibody (rabbit anti human polyclonal antibody, cat. No A0082, Dako, Glostrup, Denmarc) was diluted 1:150 and incubated with the tissue samples overnight in a humidity chamber at 4°C. After rinsing the samples with tris-buffered saline, they were incubated with biotinylated horse anti-mouse immunoglobulin G secondary antibody (Vector Lab. Inc., Burlingame; CA; USA) for 30 min. This was followed by incubation with an avidin biotin complex (ABC Kit, Vectors Laboratories, Burlingame, CA, USA) linked with alkaline phosphatase as a reporter enzyme for 50 min. Staining was achieved with neurofuchsin as a chromogen (Dako ChemMate, Dako A/S, Denmarc). Tissues where counterstained with Harris haematoxylin, dehydrated, and mounted in a xylol-soluble mount (Vitroclud, R Langenbrinck, Emmendingen, Germany).
Vessels were quantified in 17 representative regions of interest (each 0.091 mm²) using a digital video analysis system and vessel density/mm² was assessed.
For myofibroblast detection, longitudinal sections were deparaffinized, hydrated and non-specific antibody binding was blocked with 10% normal horse serum (Vector Laboratories Inc., Burlingame, CA, USA). Afterwards mouse anti-human ASMA monoclonal antibody (mouse anti human monoclonal antibody, cat. No M0851, Dako, Glostrup, Denmarc) was applied in a dilution of 1:100 over night at 4°C in a humidity chamber. All subsequent steps were identical to those described above for factor VIII staining. Quantification was conducted using a digital video analysis system and the number of myofibroblast/mm² was assessed in 10 defined regions of interest (each 0.364 mm²).
Statistical analysis
A Shapiro-Wilks Test was performed to evaluate the distribution of all parameters of interest. Due to non-parametric distribution, an initial Kruskal Wallice test for group comparison was performed followed by a Mann–Whitney U test for pairwise comparisons of cellularity, myofibroblast density and vascular density between Ebeam sterilized grafts, fresh frozen allografts, autografts, native flexor tendons and native ACL. The analysis of biomechanical parameters was conducted in the same manner, but without the flexor tendon control group. Results were corrected with Bonferroni-Holm correction. Level of significance was set at P < 0.05.
Results
Two animals were lost, one because of complications during the operation and one because of a cortico-cerebral necrosis which developed 2 month after the operation. To maintain the appropriate number of specimens, two additional sheep were included in the study. Immediately after surgery, touch loading of the operated extremity was seen in all animals. Full load bearing in Ebeam group was seen on average after 19 days whereas in fresh frozen allograft group full load bearing was seen already 12 days post-surgery. At time of sacrifice, all animals had free range of motion. At 6 weeks, three animals of the Ebeam group showed a moderate effusion. At 12 weeks, two animals of the Ebeam group and one animal of the fresh frozen allograft group also had a moderate effusion. Load bearing was limited in these animals. In one animal in the Ebeam group, the graft was resorbed and samples could only be harvested at the tibial insertion for histological analysis. In the other animals, no signs of inflammations or fibrotic changes were observed.
Decreased biomechanical properties in Ebeam treated grafts (Fig. 1)
Compared to the native ACL, failure load (LTF) and stiffness were significantly decreased in all groups (LTF: P = 0.014; 0.013; 0.027, stiffness: P = 0.015; 0.03; 0.024) at both time points. AP-laxity was significantly decreased after 6 weeks in Ebeam treated group and autograft group compared to native ACL (P = 0.015, 0.042).
After 6 weeks, three grafts failed in the Ebeam group before testing was finished, so total failure load could not be evaluated. Biomechanical testing showed decreased failure load, stiffness and AP-laxity, in the Ebeam group compared to the fresh frozen allograft group and a significant decreased failure load compared to autograft group (P = 0.024, Table 1; Fig. 1). In the Ebeam group, stiffness and failure load decreased from 6 to 12 weeks and AP-laxity increased. Contrary to this, failure load increased in fresh frozen allografts (FFA), while stiffness and AP-laxity stayed nearly constant. In autograft group, all the aforementioned parameters increased.
After 12 weeks, again three grafts in the Ebeam group failed before failure load testing could be completed. Failure mode in these cases was intratendinous rupture during the AP-laxity test.
Stiffness and LTF were significantly lower in the Ebeam group compared to non-irradiated allograft group (P = 0.033; 0.036) and autograft group (P = 0.024; 0.027).
Increased remodeling activity from 6 to 12 weeks in Ebeam treated grafts (Figs. 2, 3, 4, 5)
After 6 weeks, necrosis was dominant in both allograft groups. An initial repopulation in the subsynovial regions was present. This seemed to be more prominent in the Ebeam group. Cells were predominantly spindle shaped with few ovoid cells and collagen orientation was mainly parallel. In regions that showed greater repopulation, collagen orientation was more irregular.
Histologies of the H&E staining of Autograft, FFA and Ebeam group, original magnification ×100. 6 weeks (a–c): cellularity was highest in the autograft group (a). In Ebeam group a beginning repopulation was visible in the subsynovial parts of the graft (c) whereas necrosis was dominant in fresh frozen allografts (b). 12 weeks (d–f): Ebeam treated grafts (f) showed an increased cellularity compared to both other groups. Collagen orientation was more irregular in Ebeam group treated compared to autograft group (d). (Color figure online)
Histologies of the alpha-smooth-muscle-actin staining, original magnification ×200, 6 weeks (top): autografts showed highest myofibroblast density (a) followed by Ebeam group (c) and FFA group (b) 12 weeks: 8 (down): myofibroblast density was significantly increased in Ebeam group (f) compared to FFA group (e) and increased compared to autograft group (d). (Color figure online)
Histologies of the vascularity of FFA and Ebeam group during the early remodeling, von-willebrandt-factor staining, original magnification ×100. 6 weeks (top): in some Ebeam treated grafts (b) vessel ingrowth up to intermediate regions of the graft was visible whereas in FFA group (a), only few vessels were visible subsynovial. From 6 to 12 weeks vascularity increased in both groups. 12 weeks (down): Vessel density was clearly increased in Ebeam treated grafts (d) compared to fresh frozen allografts (c)
In the autograft group higher cell and vessel density compared to the Ebeam and non-irradiated allograft groups were observed. In the periphery of the graft, intense cell and vessel ingrowth from the synovial membrane was visible. Cell distribution was non-uniform and central parts of the grafts showed a dominating necrosis. Cell shape was mainly ovoid and collagen alignment was irregular.
After 12 weeks the Ebeam and non-irradiated allograft groups showed a comparable irregular cell distribution and cell morphology with mostly spindle shaped and some ovoid cells, but an increased repopulation and cell density also in central regions of the grafts was observed in the Ebeam group. Only few lymphocytes were visible in Ebeam group, whereas in the non-treated allograft group, infiltration with inflammatory cells such lymphocytes and granulocytes was visible. Collagen orientation was mainly irregular in all parts of the grafts.
Cell and vessel distribution in the autograft group were uniform in all parts of the graft with a higher cellularity and vascularity compared to native controls. Cell shape was mainly ovoid with some spindle shaped cells. Collagen orientation was regular.
Increased repopulation and myofibroblast activity after 12 weeks in Ebeam treated grafts
Semiquantitative investigations after 6 weeks showed decreased cell and myofibroblast (Table 2; Figs. 2a, b, 3, 4) density in both allograft groups in comparison to those in the autograft group, the hamstring tendon control group (HAT) and the intact ACL group. When compared with the non-irradiated allograft group, myofibroblast density in the Ebeam group was nearly twice as high, but was half of that found in the autograft group.
In the non-irradiated allograft group, cell density was significantly decreased compared with intact ACLs (P = 0.007). From 6 to 12 weeks, cell density increased significantly in all groups (P = 0.01; 0.036). Myofibroblast density also increased in all groups with a significant increase in the Ebeam treated group (P < 0.001).
After 12 weeks, cell density in the Ebeam treated group was higher compared to all other groups and controls although no significance was found.
Myofibroblast density was significantly increased in the Ebeam treated group compared to non-irradiated allograft group (P = 0.01) and HAT (P = 0.02) and nearly twice as high compared to the autograft group.
Increased revascularization in Ebeam treated grafts compared to fresh frozen allografts
Vessel density (Table 2; Fig. 2c, 5) was substantially elevated in Ebeam treated group at both time points compared to non-irradiated allograft group and the control groups. Compared to autograft group, vessel density was lower in Ebeam treated grafts at 6 weeks but increased after 12 weeks.
From 6 to 12 weeks vessel density increased in both allograft groups with a significant increase in the non-irradiated allograft group (P < 0.001) but decreased in the autograft group.
After 12 weeks, vessel density was significantly increased in Ebeam treated group compared to native controls (P < 0.001; 0.019) and nearly twice as high as that in the non-irradiated allograft and autograft group although no significance was found.
Discussion
The use of allograft tissue for ACL reconstruction has substantially increased during recent years. However, although donor screening and blood testing has reduced the risk of disease transmission from non-sterilized allograft tissue, the risk has not been eliminated (Buck et al. 1989; Simonds et al. 1992; Kainer et al. 2004; Kainer and Jarvis 2004; Update 2002).
For this reason many suppliers of allograft tissue transplants employ validated sterilization methods (Cohen and Sekiya 2007; McAllister et al. 2007). Indeed, in some countries, for certain classes of transplants including tendons and ligaments, this is mandated by law. Gamma irradiation is perhaps the most widely applied method of sterilizing tissue transplants. However, at the dose required for sufficient pathogen inactivation (>30 kGy) (Pruss et al. 2002; Fideler et al. 1994), it is known to have inacceptable adverse effects (McGilvray et al. 2010; Rappe et al. 2007; Gibbons et al. 1991; Fideler et al. 1995; Salehpour et al. 1995; Sun et al. 2009; Curran et al. 2004).
Electron Beam irradiation is a promising alternative to gamma irradiation since it can be operated as a fast throughput system (Seto et al. 2008) with better control of the sterilization environment and better control of dosage than is possible with conventional gamma sterilization plants. Ebeam sterilization showed promising results in our in vitro biomechanical analyses (Hoburg et al. 2010). Moreover, we were able to optimize the radiation process by performing it under low oxygen conditions and at low temperature. This should reduce undesirable oxygen radical induced secondary reactions and therefore mitigate some of the damaging effects of irradiation on material properties (Dziedzic-Goclawska et al. 2005).
Based on these findings and the lack of in vivo studies on the effect of Ebeam irradiation, we planned this study to investigate the biological remodeling of Ebeam irradiated allografts under the hypothesis that Ebeam treated allografts would show comparable remodeling and revascularization and uncompromised biomechanical properties compared to fresh frozen allografts and autografts.
The results of this study suggest that our hypothesis must be rejected. Ebeam sterilization significantly altered allograft remodeling. Ebeam treated grafts showed an increased cellular repopulation and neovascularization, which was most pronounced at 12 weeks. This resulted in a significant reduction of the graft’s biomechanical strength up to 12 weeks. There was no recovery of biomechanical function during the early remodeling from 6 to 12 weeks of healing. Other authors analyzing the influence of gamma irradiation made similar observations. They also found an initial loss of biomechanical strength, paralleled by severe vascular budding. With ensuing time, differences disappeared at 52 weeks between gamma irradiated and fresh frozen allografts (Goertzen et al. 1995).
However, since no time points later than 12 weeks were assessed in this study, no conclusions can be drawn about whether recovery of mechanical and biological function might have occurred with longer time points. The lack of biological and mechanical recovery of Ebeam irradiated allografts from 6 to 12 weeks is a major concern, since it has been shown that auto- as well as allografts follow a similar path of morphological and functional recovery from 6 to 12 and to 52 weeks. Today’s rehabilitation programs aim to increase loading at 6 weeks after ACL reconstruction to restore normal lower extremity function. A decrease in graft strength during this time period would possibly lead to graft elongation or even early graft failure, therefore posing an unacceptable risk for safe functional recovery of patients.
In a previous in vivo animal study in rats (Mae et al. 2003), mechanical function of gamma irradiated grafts returned to that of non-irradiated allograft after 6 months of healing, even though substantial differences existed during the early healing process. They found an initial decrease of tensile strength up to 4 weeks but contrary to our study this was followed by a gradual increase in tensile strength in the irradiated allograft group. In the current study, biomechanical strength decreased from 6 to 12 weeks in Ebeam treated grafts. The deviating results might be a consequence of the different animal and surgical models used in these studies and the irradiation dose. There are differences in mechanical loading and remodeling depending on the animal model. Furthermore, grafts in their study were treated with 25 kGy gamma.
A secondary aim of our study was to indentify reasons for biomechanical failure of irradiated grafts.
It is known that two main mechanisms are involved in irradiation associated pathogen inactivation. First, energy from ionizing radiation is directly transferred to the pathogen and second, ionizing radiation leads to the generation of free radicals as a result of water radiolysis. Both effects cause damage of the pathogen’s DNA/RNA including single and double-strand breaks, intra-strand cross-links and damage to other parts of the nucleic acids (Dziedzic-Goclawska et al. 2005). Based on this information, one theory assumes that the impairment of the biomechanical properties of soft-tissue tendons is caused by collagen cross-link breaks, which weakens the collagen network (Gouk et al. 2008; Seto et al. 2008; Salehpour et al. 1995; Cheung et al. 1990).
Since we did not find significant differences in the ratio of non- reducible collagen crosslinks nor in crimp-pattern between Ebeam irradiated and fresh frozen allografts (unpublished data), we postulate that the increased remodeling and revascularization may have produced structural changes that provoked biomechanical failure.
It has been shown in other studies that an increased vessel density leads to a reduction of biomechanical strength in soft-tissue grafts (Weiler et al. 2001; Yoshikawa et al. 2006).
This confirms the results of other researchers, who also found decreased mechanical properties accompanied with increased remodeling activity after gamma irradiation in vitro (Gouk et al. 2008) and in vivo (Gorschewsky et al. 2002). Gorschewsky et al. found significantly higher failure rates in his clinical trial comparing autografts and gamma irradiated allografts. They concluded, that an ongoing hypervascularization accompanied with an irregular collagen orientation decreased the mechanical function of allografts.
In the current study and in a previous in vivo study that we conducted using an identical animal model, non-irradiated fresh-frozen allografts showed an increased vessel density and biomechanical strength from 6 to 12 weeks (Scheffler et al. 2008b). This was in contrast to the current results of the Ebeam irradiated grafts showing an increase in vascularity and cellularity and a loss of mechanical strength from 6 to 12 weeks of healing. Compared to non-irradiated allograft the increase in vessel and cell density was markedly pronounced, and both values were increased in Ebeam treated grafts after 6 and 12 weeks. We assume that the overshooting revascularization and repopulation in Ebeam treated grafts might be one explanation for the different biomechanical results. However, we suspect that the irradiation procedure must have caused other adverse effects. Further studies are necessary to clarify these effects. Furthermore, this study was only able to report on differences between irradiated and non-irradiated allografts. It was not possible to provide details on the actual pathomechanisms that trigger the changes observed in this study. Further analyses are necessary to examine and clarify the mechanisms that caused the increased remodeling. We found neither inflammatory reactions nor infections in our Ebeam irradiated allografts. However, we have only analyzed the grafts using H&E staining and this does not allow for differentiation between inflammatory cell types, cytokines and other factors. An improved understanding of the direct consequences of soft-tissue irradiation might allow for the development of protective measures to allow safe irradiation without biomechanical and biological disadvantages.
In summary, even though Ebeam irradiation showed promising results in vitro, it cannot be recommended in its current form for terminal soft-tissue graft sterilization, due to its adverse effects on early biological healing and subsequent impairment of biomechanical function, when used in ACL reconstruction.
References
Allen MJ, Houlton JE, Adams SB, Rushton N (1998) The surgical anatomy of the stifle joint in sheep. Vet Surg 27(6):596–605
Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr (2008) Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank 9(4):289–298
Buck BE, Malinin TI, Brown MD (1989) Bone transplantation and human immunodeficiency virus. An estimate of risk of acquired immunodeficiency syndrome (AIDS). Clin Orthop Relat Res 240:129–136
Cheung DT, Perelman N, Tong D, Nimni ME (1990) The effect of gamma-irradiation on collagen molecules, isolated alpha-chains, and crosslinked native fibers. J Biomed Mater Res 24(5):581–589
Cohen SB, Sekiya JK (2007) Allograft safety in anterior cruciate ligament reconstruction. Clin Sports Med 26(4):597–605
Curran AR, Adams DJ, Gill JL, Steiner ME, Scheller AD (2004) The biomechanical effects of low-dose irradiation on bone-patellar tendon-bone allografts. Am J Sports Med 32(5):1131–1135
Dziedzic-Goclawska A, Kaminski A, Uhrynowska-Tyszkiewicz I, Stachowicz W (2005) Irradiation as a safety procedure in tissue banking. Cell Tissue Bank 6(3):201–219
Fideler BM, Vangsness CT Jr, Moore T, Li Z, Rasheed S (1994) Effects of gamma irradiation on the human immunodeficiency virus. A study in frozen human bone-patellar ligament-bone grafts obtained from infected cadavera. J Bone Joint Surg Am 76(7):1032–1035
Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T (1995) Gamma irradiation: effects on biomechanical properties of human bone-patellar tendon-bone allografts. Am J Sports Med 23(5):643–646
Gerbi BJ, Antolak JA, Deibel FC, Followill DS, Herman MG, Higgins PD, Huq MS, Mihailidis DN, Yorke ED, Hogstrom KR, Khan FM (2009) Recommendations for clinical electron beam dosimetry: supplement to the recommendations of Task Group 25. Med Phys 36(7):3239–3279
Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR (1991) Effects of gamma irradiation on the initial mechanical and material properties of goat bone-patellar tendon-bone allografts. J Orthop Res 9(2):209–218
Goertzen MJ, Clahsen H, Burrig KF, Schulitz KP (1995) Sterilisation of canine anterior cruciate allografts by gamma irradiation in argon. Mechanical and neurohistological properties retained one year after transplantation. J Bone Joint Surg Br 77(2):205–212
Gorschewsky O, Browa A, Vogel U, Stauffer E (2002) Clinico-histologic comparison of allogenic and autologous bone-tendon-bone using one-third of the patellar tendon in reconstruction of the anterior cruciate ligament. Unfallchirurg 105(8):703–714
Gouk SS, Lim TM, Teoh SH, Sun WQ (2008) Alterations of human acellular tissue matrix by gamma irradiation: histology, biomechanical property, stability, in vitro cell repopulation, and remodeling. J Biomed Mater Res B Appl Biomater 84(1):205–217
Harner CD, Olson E, Irrgang JJ, Silverstein S, Fu FH, Silbey M (1996) Allograft versus autograft anterior cruciate ligament reconstruction: 3- to 5-year outcome. Clin Orthop 324:134–144
Hoburg AT, Keshlaf S, Schmidt T, Smith M, Gohs U, Perka C, Pruss A, Scheffler S (2010) Effect of electron beam irradiation on biomechanical properties of patellar tendon allografts in anterior cruciate ligament reconstruction. Am J Sports Med 38(6):1134–1140
Hunt P, Scheffler SU, Unterhauser FN, Weiler A (2005) A model of soft-tissue graft anterior cruciate ligament reconstruction in sheep. Arch Orthop Trauma Surg 125(4):238–248
Jackson DW, Windler GE, Simon TM (1990) Intraarticular reaction associated with the use of freeze-dried, ethylene oxide-sterilized bone-patella tendon-bone allografts in the reconstruction of the anterior cruciate ligament. Am J Sports Med 18(1):1–10 discussion 10–11
Kainer MA, Jarvis WR (2004) HIV-1 and HCV infections among antibody-negative blood donors. N Engl J Med 351(21):2232–2235 author reply 2232–2235
Kainer MA, Linden JV, Whaley DN, Holmes HT, Jarvis WR, Jernigan DB, Archibald LK (2004) Clostridium infections associated with musculoskeletal-tissue allografts. N Engl J Med 350(25):2564–2571
Krych AJ, Jackson JD, Hoskin TL, Dahm DL (2008) A meta-analysis of patellar tendon autograft versus patellar tendon allograft in anterior cruciate ligament reconstruction. Arthroscopy 24(3):292–298
Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T (2003) Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop Relat Res 414:305–314
McAllister DR, Joyce MJ, Mann BJ, Vangsness CT Jr (2007) Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med 35(12):2148–2158
McGilvray KC, Santoni BG, Turner AS, Bogdansky S, Wheeler DL, Puttlitz CM (2010) Effects of (60)Co gamma radiation dose on initial structural biomechanical properties of ovine bone-patellar tendon-bone allografts. Cell Tissue Bank. doi:10.1007/s10561-010-9170-z
Nemzek JA, Arnoczky SP, Swenson CL (1994) Retroviral transmission by the transplantation of connective-tissue allografts. An experimental study. J Bone Joint Surg Am 76(7):1036–1041
Pruss A, Kao M, Gohs U, Koscielny J, von Versen R, Pauli G (2002) Effect of gamma irradiation on human cortical bone transplants contaminated with enveloped and non-enveloped viruses. Biologicals 30(2):125–133
Radford WJP, Amis AA, Stead AC (1996) The ovine stifle as a model for human cruciate ligament surgery. Vet Comp Orthop Traumatol 9:134–139
Rappe M, Horodyski M, Meister K, Indelicato PA (2007) Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med 35(10):1653–1658
Reid BD (1998) The Sterways process: a new approach to inactivating viruses using gamma radiation. Biologicals 26(2):125–129
Roberts TS, Drez D Jr, McCarthy W, Paine R (1991) Anterior cruciate ligament reconstruction using freeze-dried, ethylene oxide-sterilized, bone-patellar tendon-bone allografts. Two year results in thirty-six patients. Am J Sports Med 19(1):35–41
Salehpour A, Butler DL, Proch FS, Schwartz HE, Feder SM, Doxey CM, Ratcliffe A (1995) Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone-patellar tendon-bone allografts. J Orthop Res 13(6):898–906
Scheffler S, Trautmann S, Smith M, Kalus U, von Versen R, Pauli G, Pruss A (2007) No influence of collagenous proteins of Achilles tendon, skin and cartilage on the virus-inactivating efficacy of peracetic acid-ethanol. Biologicals 35(4):355–359
Scheffler SU, Gonnermann J, Kamp J, Przybilla D, Pruss A (2008a) Remodeling of ACL allografts is inhibited by peracetic acid sterilization. Clin Orthop Relat Res 466(8):1810–1818
Scheffler SU, Schmidt T, Gangey I, Dustmann M, Unterhauser F, Weiler A (2008b) Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy 24(4):448–458
Seitz H, Hausner T, Schlenz I, Lang S, Eschberger J (1997) Vascular anatomy of the ovine anterior cruciate ligament. A macroscopic, histological and radiographic study. Arch Orthop Trauma Surg 116(1–2):19–21
Seto A, Gatt CJ Jr, Dunn MG (2008) Radioprotection of tendon tissue via crosslinking and free radical scavenging. Clin Orthop Relat Res 466(8):1788–1795
Simonds RJ, Holmberg SD, Hurwitz RL, Coleman TR, Bottenfield S, Conley LJ, Kohlenberg SH, Castro KG, Dahan BA, Schable CA (1992) Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med 326(11):726–732
Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB (2009) ACL reconstruction with BPTB autograft and irradiated fresh frozen allograft. J Zhejiang Univ Sci B 10(4):306–316
Unterhauser FN (2004) Revaskularisierung und Nachweis von Myofibroblasten im freien Sehnentransplantat nach vorderem Kreuzbandersatz—Histologische 2-Jahres Untersuchung am Schaf. Tierexperimentelle Langzeitstudie, Medizinische Fakultät—Universitätsmedizin Berlin, Berlin
Update (2002) Update: allograft associated bacterial infections-United States. vol 51. MMWR Morb Mortal Wkly Rep
Weiler A, Peters G, Maurer J, Unterhauser FN, Sudkamp NP (2001) Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A two-year study in sheep. Am J Sports Med 29(6):751–761
Weiler A, Peters G, Maurer J, Unterhauser FN, Sudkamp NP (2002) Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A two-year study in sheep. Am J Sports Med 29(6):751–761
Yoshikawa T, Tohyama H, Katsura T, Kondo E, Kotani Y, Matsumoto H, Toyama Y, Yasuda K (2006) Effects of local administration of vascular endothelial growth factor on mechanical characteristics of the semitendinosus tendon graft after anterior cruciate ligament reconstruction in sheep. Am J Sports Med 34(12):1918–1925
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt, T., Hoburg, A., Broziat, C. et al. Sterilization with electron beam irradiation influences the biomechanical properties and the early remodeling of tendon allografts for reconstruction of the anterior cruciate ligament (ACL). Cell Tissue Bank 13, 387–400 (2012). https://doi.org/10.1007/s10561-011-9289-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-011-9289-6