Abstract
Purpose
The present study was to determine whether OP2113 could limit myocardial infarction size and the no-reflow phenomenon in a rat myocardial ischemia/reperfusion model.
Methods
Rat heart–isolated mitochondria (RHM) were used to investigate mitochondrial respiration and mitochondrial reactive oxygen species (mtROS) generation both in normal conditions and in ischemia/reperfusion-mimicking conditions (using high concentrations of succinate). Human skeletal muscle myoblasts (HSMM) in culture were used to investigate the cellular intermittent deprivation in energy substrates and oxygen as reported in ischemia/reperfusion conditions. In vivo, rats were anesthetized and subjected to 30 min of left coronary artery occlusion followed by 3 h of reperfusion. Rats were randomized to receive OP2113 as an intravenous infusion starting either 5 min prior to coronary artery occlusion (preventive), or 5 min prior to reperfusion (curative), or to receive vehicle starting 5 min prior to coronary artery occlusion. Infusions continued until the end of the study (3 h of reperfusion).
Results
RHM treated with OP2113 showed a concentration-dependent reduction of succinate-induced mtROS generation. In HSMM cells, OP2113 treatment (5–10 μM) during 48H prevented the reduction in the steady-state level of ATP measured just after reperfusion injuries and decreased the mitochondrial affinity to oxygen. In vivo, myocardial infarct size, expressed as the percentage of the ischemic risk zone, was significantly lower in the OP2113-treated preventive group (44.5 ± 2.9%) versus that in the vehicle group (57.0 ± 3.6%; p < 0.05), with a non-significant trend toward a smaller infarct size in the curative group (50.8 ± 3.9%). The area of no reflow as a percentage of the risk zone was significantly smaller in both the OP2113-treated preventive (28.8 ± 2.4%; p = 0.026 vs vehicle) and curative groups (30.1 ± 2.3%; p = 0.04 vs vehicle) compared with the vehicle group (38.9 ± 3.1%). OP2113 was not associated with any hemodynamic changes.
Conclusions
These results suggest that OP2113 is a promising mitochondrial ROS–modulating agent to reduce no-reflow as well as to reduce myocardial infarct size, especially if it is on board early in the course of the infarction. It appears to have benefit on no-reflow even when administered relatively late in the course of ischemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite advances in reperfusion therapy for acute ST elevation myocardial infarction, in hospital, mortality in some areas of the USA is as high as 8% [1]. In addition, heart failure remains a significant complication of myocardial infarction [2]. A potential therapy that might further improve outcomes following myocardial infarction is to better protect the energy-producing factories of the cell: the mitochondria.
Anethole trithione (ATT, also named 5-(4-methoxyphenyl)-3H-1,2-dithiole-3-thione) has been marketed in many countries and used in human therapy as an antioxidant agent to decrease mitochondrial reactive oxygen species (ROS/H2O2) production (for details about the drug, read ref [3]). OP2113 is a formulation of pure 0.2 mg/kg ATT included in sulfobutylether-β-cyclodextrin (SBE-CD) 10% to improve the solubilization of the compound compatible with an intravenous (IV) administration. Furthermore, SBE-CD 10% was chosen for its lack of effect on the hemodynamics. The active ingredient ATT in OP2113 is a suppressor of mitochondrial complex I superoxide/H2O2 production [3, 4], which may have cardioprotective properties in the setting of acute myocardial ischemia/reperfusion. ATT has already shown promise in reducing ischemia/reperfusion injury in an isolated rat heart model of global ischemia (30 min) and reperfusion (120 min) when the drug was started prior to ischemia. In this model, ATT significantly improved left ventricular (LV) function during reperfusion and reduced infarct size [3]. In a similar model, when ATT treatment was started at the time of reperfusion, it did reduce the release of creatine phosphokinase (CPK) during reperfusion, suggesting a reduction in cell death, but did not improve cardiac contractility during reperfusion (Thierry Sulpice, Cardiomedex, Toulouse, unpublished data). There is a first preliminary series of studies examining ATT in an in vivo model of experimental myocardial infarction using the sheep as the experimental model (OP2 Drugs in partnership with INSERM Unit 1045, Bordeaux, Paris, unpublished data). The available results demonstrated that the drug reduced ECG manifestations of infarction and reduced anatomical infarct size whether it was given in a preventive (prior to occlusion) or curative (given at reperfusion) fashion. Whether ATT could consistently reduce anatomic myocardial infarct size as well as the anatomic size of the no-reflow zone in a standardized rat model of myocardial infarction/reperfusion, given in a prophylactic (preventive) manner or prior to reperfusion (curative manner), is not known. Our present study investigated the mode of action of this drug on the potential prevention of mitochondrial ROS production in 2 in vitro experiments and determined the effects of OP2113, an IV formulation containing ATT, on anatomic myocardial infarct size and no-reflow in an acute rat ischemia/reperfusion model.
Methods
All experimental protocols were approved by the Institutional Animal Care and Use Committee, and performed in accordance with the “Guide for the Care and Use of Laboratory Animals” (NIH publication no. 85-23, National Academy Press, Washington DC, revised 2011). The Huntington Medical Research Institutes is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
High-Resolution Respirometry Coupled to Fluorometry for the Measurement of Mitochondrial Respiration and mtROS
Mitochondria were isolated from male Sprague Dawley rat hearts using our established methods [5]. Respiratory control ratios (RCRs) were measured using glutamate/malate (10 mM/2 mM respectively) and ADP (5 mM) as energy substrates (37 °C) for each isolation by calculating state 3/state 4 rates of respiration [6, 7]. If RCRs were less than 6, the preparation was not used. Mitochondrial function was determined using previously established protocols [8, 9]. Briefly, we simultaneously measured oxygen consumption and H2O2 production via high-resolution respirometry using the Oxygraph-2ks (“O2ks”; Oroboros Instruments, Austria). All experiments were performed at 37 °C in Buffer Z (105 mM K-MES, 30 mM KCl, 10 mM KH2PO4, 5 mM MgCl2, 20 mM creatine, 1 mM EGTA, 0.05% BSA, and pH 7.1) with 10 μM Amplex Red (AmR; Thermo Fisher Scientific), 4 U/ml horseradish peroxidase (HRP), and 30 U/ml superoxide dismutase added for detection of reactive oxygen species (ROS) production. H2O2 calibrations were performed using 0.1-μM titrations to generate a standard curve.
A subset of mitochondria were allowed to incubate in the O2k chamber for 15 min with varying concentrations of OP2113 (n = 8) or a vehicle control (n = 32 pooled, one control per condition in each well of the 2-well O2ks) prior to assessing the ROS produced by succinate-induced reverse electron transfer (RET). RET was induced by the addition of 10 mM succinate to isolated mitochondria in state 4 conditions, and ROS production was monitored for 7 min followed by the addition of 0.5 μM rotenone to inhibit complex I. After both respiration and H2O2 production rates reached a steady state, 5 mM malonate was added to inhibit complex II–dependent respiration. Throughout the protocol, rates of mitochondrial reactive oxygen species (mtROS) production and respiration were analyzed by averaging the data points collected every 2 s over 1 min. For succinate-induced RET-H2O2 production and respiration, the same data sampling interval was used but the epoch of time was extended to 5 min. ROS production values were also normalized to simultaneous rates of O2 consumption for further analysis.
Oxygen Kinetics and P50 Determinations
Respiration of suspended cells was measured at 37 °C by high-resolution respirometry with the OROBOROS O2k Oxygraph with chamber volumes set at 2 ml. The software DatLab (OROBOROS, Innsbruck, Austria) was used for data acquisition (1-s time intervals) and analysis, including calculation of the time derivative of oxygen concentration, signal deconvolution dependent on the response time of the oxygen sensor, and correction for instrumental background oxygen flux [10]. Routine respiration in culture medium and oligomycin-inhibited respiration were measured in the oxygen range of 90–140 μM. Finally, respiration was inhibited by sequential addition of rotenone (to test for the effect of inhibiting complex I activity) and antimycin A (inhibiting complex III). In some cases, oxygen levels were increased intermittently by partial re-aeration. This titration protocol was completed within 50 min. The oxygen solubility of the medium was 9.4 μM/kPa. Chemicals were purchased from Sigma.
Human Skeletal Muscle Myoblast Cell Culture and In Vitro Ischemia/Reperfusion Protocol
Human skeletal muscle myoblast (HSMM) cells were obtained from Lonza and grown in the SkGM2 Skeletal Muscle Cell Growth Medium-2 with supplements (Lonza CC-3244) required for growth of skeletal muscle myoblasts. The ischemia/reperfusion (I/R) in vitro protocol was performed as follows. At the beginning of the ischemic phase, the SkGM medium was replaced by a balanced salt solution (PBS) that contained no energy substrate and the cells were placed in a hypoxic incubator (Xvivo System (BioSpherix, Ltd., USA)) for 1 h. Then, for reperfusion, the cells were replaced at atmospheric oxygen in the SkGM2 medium containing energy substrates.
ATP Measurements
The intracellular ATP content was measured using a bioluminescent ATP kit (CellTiter-Glo, Promega). Cells were seeded in 96-well plates (20,000 cells by well). Following reperfusion, the HSMM cells were immediately lysed to release the intracellular ATP by adding a lysis buffer containing luciferin and luciferase for 10 min at room temperature. ATP concentration was determined with the light-emitting luciferase-catalyzed oxidation of luciferin with ATP. The bioluminescence was measured (1-s integration time) on a luminometer (CLARIOstar, BMG LABTECH). Standardization was performed with known quantities of standard ATP measured under the same conditions.
In Vivo Myocardial Ischemia/Reperfusion Experiments in Rats
Female Sprague Dawley rats (~ 6 months old) were anesthetized with intraperitoneal ketamine (90 mg/kg) and xylazine (10 mg/kg). Only female rats were used in this study as we have previously reported that gender had no effect on the extent of myocardial injury in the setting of experimental myocardial infarction in rats [11]. We also observed that lethal reperfusion-induced arrhythmias led to a higher mortality in male rats versus female rats [12]. The animals were intubated and mechanically ventilated with room air at respiratory rate 60 cycles/min and tidal volume 1 ml per 100 g body weight (Harvard Apparatus Rodent Ventilator, Model 683, South Natick, MA). A water circulating heating pad was used to keep the rat body temperature at around 37 °C. Their neck surgical area was shaved and cleaned, and catheters were inserted into the jugular vein (for drug delivery) and carotid artery (for arterial waveform monitoring) via cut downs. Under clean conditions, the chest cavity was opened through an incision in the left 4th intercostal space to expose the heart. The pericardium was gently removed to expose the anterior surface of the left ventricle. A 4-0 silk suture was placed under the proximal portion of the left coronary artery as it runs through the interventricular groove just under the tip of the left atrial appendage. The end of the suture was threaded through a small plastic tube to make a snare for coronary artery occlusion. At the time of occlusion, the artery was pulled through the plastic tube and the tube was clamped. Coronary artery reperfusion occurred by releasing the clamp and watching the surface of the heart for reactive hyperemia.
Rats were randomized into one of the 3 groups: OP2113 started at 5 min before coronary artery occlusion (preventive group), OP2113 started at 5 min prior to reperfusion (curative group), or vehicle (control group) infusion started at 5 min before coronary artery occlusion. The investigators were blinded to the animal randomization. Infusions in all groups were continuous throughout the end of the study (at 3 h of reperfusion). In treated rats, the dose of OP2113 was 0.2 mg/kg/h delivered in saline at a rate of infusion of 2 ml/kg/h. The left coronary artery was occluded for 30 min followed by 3 h of reperfusion.
At the end of 3 h of reperfusion, thioflavin S solution was injected into the jugular vein during the last 1 min of reperfusion to assess the distribution of the no-reflow phenomenon (also known as the zone of microvascular obstruction). Thioflavin S is a dye that appears fluorescent in areas receiving perfusion, when heart slices are visualized under ultraviolet (UV) light, while the no-reflow area, which lacks perfusion, appears dark (non-fluorescent). At the end of the period of reperfusion after thioflavin S injection, the proximal coronary artery was briefly re-occluded and blue dye (Super imperse blue) was injected into the jugular vein. The blue dye circulates only to perfused areas and does not reach the ischemic zone (which appears pink when viewing heart slices under standard white light). At the end of this step, the animal was euthanized under deep anesthesia with ketamine/xylazine by intravascular injection of KCl (149 mg/ml) at 0.5 ml/250 g body weight in order to stop the heart in a relative diastolic or relaxed state. The heart was excised and was gently washed in clear saline and then transected into 4 transverse slices from apex to base. The heart slices were photographed under white light in order to delineate the ischemic risk zone (pink) in contrast to the non-ischemic regions (blue) that received the blue dye. The heart slices were then photographed under UV light in order to delineate the areas of perfusion by thioflavin S (fluorescent areas) versus the no-reflow zones (non-fluorescent). Finally, the hearts were incubated in 1% of triphenyl tetrazolium chloride (TTC), a chemical that stains viable cells brick red, while dead or necrotic cells appear white. The photographs were used for planimetry in order to determine the percentage of each heart slice that was at risk, infarcted, or contained no reflow. Planimetered photos were corrected for the weight of each heart slice, and then, the percentage of the mass of each left ventricle that was at risk (ischemic), demonstrated no-reflow, and was necrotic was calculated. Infarct size was expressed as the percentage of the ischemic risk zone that went on to develop ischemic necrosis. The no-reflow zone was also expressed as a percent of the LV risk zone and also as percentage of the zone of necrosis.
Rectal temperature, heart rate, and blood pressure were monitored throughout the surgical procedure.
Statistical Analysis
All data are reported as mean ± SEM. Values between the groups were compared with one-way analysis of variance (ANOVA). Statistically significant differences were established at p < 0.05. If an ANOVA three-way comparisons was significant, then 2-way comparisons were performed using the Holm-Sidak method.
Results
Effect of OP2113 on Mitochondrial Respiration and mtROS Generation
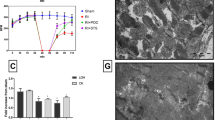
Previous studies demonstrated that ATT, the active ingredient of OP2113, acts as a suppressor of superoxide production at complex I (S1QEL) with no adverse effect on mitochondrial respiration [3, 4]. We verified first that OP2113 had no effect on the respiration of isolated rat heart mitochondria fueled with succinate, as occurs in conditions of I/R [13]. The results indicated that succinate stimulates RHM respiration as expected, and that OP2113 used at 5 and 15 μM had no effect on this parameter (Fig. 1a). The succinate-induced RET–generated H2O2 production was also measured by high-resolution respirometry coupled to fluorometry (Oroboros O2K-Fluo) and showed a significant reduction (p < 0.05) of succinate-induced mtROS production by OP2113 used at 15 μM (Fig. 1b). Normalization of the flux of H2O2 generation to the flux of oxygen consumption also showed the ROS-suppressing effect of OP2113 in RHM, suggesting that ROS suppression is not a consequence of respiratory changes (Fig. 1c).
REDOX mode of action of OP2113 in isolated rat heart mitochondria. (a) The effect of OP2113 was evaluated on mitochondrial respiration using high-resolution respirometry. (b) H2O2 production was measured in rat heart–isolated mitochondria using fluorometry coupled to high-resolution respirometry (O2k-fluo system; Oroboros). RHM were treated with OP2113 at 5 or 15 μM following mtROS induction with succinate. (c) The flux of succinate-induced mtROS generation was normalized to that of oxygen consumption using the OéK-Fluo system which measures both parameters simultaneously
Effects of OP2113 on In Vitro Ischemia/Reperfusion Injuries
To study the consequences of the mtROS-suppressing effect of OP2113 in intact cells, we used HSMM in culture. To mimic the I/R situation, we developed an in vitro protocol of cellular intermittent deprivation in energy substrates and oxygen. We observed that OP2113 treatment used at 5 and 10 μM during 48H induced an increase in the steady-state level of ATP measured just after reperfusion in HSMM cells challenged by in vitro I/R (Fig. 2a). Last, we analyzed the oxygen kinetics in HSMM cells treated with OP2113 using high-resolution respirometry, as previously described [13]. In conditions of hypoxia occurring during I/R injury, changes in the mitochondrial affinity to oxygen could promote oxidative phosphorylation. High-resolution respirometry allowed to determine the O2 tension—at which respiration operates at half of the maximal flux: P50. The P50 gives a measure of the sensitivity of the respiratory chain to changes in oxygen tension. For instance, a low P50 will allow the respiratory chain to be modulated by changes in oxygen tension at hypoxia, as observed in I/R conditions. A higher P50 will suggest an optimal regulation of the respiratory chain by oxygen at normoxia. Here, we performed oxygen kinetics analyses of human myoblasts pretreated during 48H with OP2113 to calculate the P50 or C50 when expressed in micromolar. The results revealed a decrease of this parameter in cells treated with OP2113 (Fig. 2b).
In Vivo Ischemia/Reperfusion
A total of 64 rats were used for this protocol and were randomized into one of the 3 groups: control group (n = 22), curative group (n = 20), and preventive group (n = 22). Three rats died at around 2 h after coronary artery reperfusion (1 in the control group and 2 in the preventive group). Another 3 rats were excluded due to no ischemic risk area available because the heart stopped beating when blue dye was injected (1 rat in each of the 3 groups). Hemodynamics and histological data (area at risk, and area of no-reflow and necrosis) were analyzed in a blinded manner.
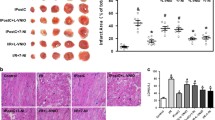
Overall statistical analysis by ANOVA showed that there was a significant difference in the area of no-reflow/area at risk (%) and the area of necrosis/area at risk (%) among the 3 groups (shown in Table 1). There was no statistically significant difference in the ischemic risk areas among the 3 groups. The areas of no-reflow occur completely within the areas of infarction, not outside those areas of irreversibly injured cardiac muscle, and were significantly smaller in the 2 treated groups compared with the control group. The infarct size was significantly reduced in the preventive group compared with the control group. There was a non-significant trend toward smaller infarct size in the curative versus the control group. Figure 4b plots the regression line of the myocardial infarct mass versus the risk zone mass and shows that for any size of the risk zone, the size of the infarct is reduced in the preventive group with a positive trend downward in the curative group compared with vehicle control. Figure 5b plots the regression line of no-reflow mass versus risk zone mass and shows that OP2113 therapy (preventive and curative) shifted the line downward, such that for any given risk zone mass, the size of the no-reflow mass is less than in the vehicle group.
Walsh et al. [14] reported that once the mean arterial pressure (MBP) reaches 55 mmHg, only a few minutes of exposure is associated with myocardial injury. In order to determine whether MBP < 55 mmHg could impact the myocardial necrosis and no-reflow size in this study, the rats in the control group were divided into subgroups based on the baseline (prior to coronary artery occlusion) MBP: MBP > 55 mmHg group and MBP < 55 mmHg group. As shown in Table 2, there was no difference in infarct and no-reflow size between the 2 subgroups.
Hemodynamics, including heart rate and arterial blood pressure, were reported at 4 time points in Table 3: (1) prior to coronary artery occlusion (baseline); (2) prior to coronary artery reperfusion (at the end of 30 min of coronary artery occlusion); (3) at 1 min after coronary artery reperfusion; and (4) at the end of 3 h of coronary artery reperfusion. There were no differences in heart rate and arterial blood pressure among the 3 groups during the procedure.
Discussion
The present study made the following key observations: Firstly, the effect of OP2113 was investigated at four mechanistic levels: (1) Mitochondrial respiration measured by high-resolution respirometry on RHM showed that OP2113 had no toxic effect on this parameter. (2) Mitochondrial H2O2 generation induced by succinate on RHM, as observed in conditions of ischemia-reperfusion, showed that OP2113 15 μM reduced mtROS generation. (3) Cellular ATP production under in vitro I/R protocol on HSMM showed a protective bioenergetic effect of OP2113. (4) Mitochondrial affinity to oxygen (P50) measured on HSMM by high-resolution respirometry showed a protective bioenergetic effect of OP2113 to respire under hypoxic conditions.
Secondly in an in vivo model, when OP2113 was administered in a preventive fashion, that is given prior to coronary artery occlusion in this acute myocardial infarct model in rats, it significantly reduced both the size of the no-reflow zone (by 26%) and the size of the myocardial infarction (by 22%). When OP2113 was given in a curative fashion, just shortly before reperfusion, it significantly reduced the size of the no-reflow zone (by 23%) but it did not significantly reduce the size of the myocardial infarction. There was a trend toward a modest non-statistically significant reduction in infarct size with curative treatment (by 11%). The benefit of OP2113 was observed without effect on hemodynamics.
The mitochondria play a crucial role in supplying ATP, but also are a source of deleterious reactive oxygen species that are generated upon ischemia/reperfusion injury. There is interest in protecting the mitochondria during ischemia and reperfusion as one approach to further reduce myocardial infarction [15]. Previous studies have tried to protect the mitochondria during phases of injury by protecting cardiolipin and stabilizing the configuration of the electron transport chain, or by inhibiting the mitochondrial permeability transition pore from opening during ischemia and reperfusion, and by opening the mitochondrial KATP channel. Other studies attempted to scavenge reactive oxygen species that formed during the early phase of myocardial ischemia/reperfusion. Experimental studies showed promise with these agents, but their clinical trials were in general disappointing [16]. Recent studies demonstrated that mitochondrial respiratory chain—and specifically complex I—damages occur during ischemia and reperfusion, and complex I appears to play a central role in the mitochondrial ROS production in ischemia/reperfusion injury [17]. Most of the experimental data [18, 19] support the view that a specific subunit of complex 1 called flavin mononucleotide (FMN) is the source for superoxide/H2O2 in complex I operating in the ischemia/reperfusion model. It is now well established that succinate accumulates several-fold in the ischemic heart and is then rapidly oxidized upon reperfusion, contributing to reactive oxygen species (ROS) production by mitochondria through the activation of the complex 1 [20]. ATT is a unique compound in that it interacts with the mitochondrial complex I to actually suppress the production of superoxide/H2O. Dias Amoedo et al. [4] showed that ATT was docked within the NADH dehydrogenase [ubiquinone] flavoprotein 1 (NDUFV 1) core subunit of complex I that contains one iron-sulfur cluster ([4Fe-4S]; “SF4”) and the FMN cofactor. This specific interaction with FMN leads to a deactivation of the ischemia/reperfusion mode of complex 1 and to an immediate blockade of the ROS overproduction. Therefore, the present results confirmed that blockade of the mtROS generated at complex I through succinate-induced RET with OP2113 led to a significant reduction of mtROS production. Furthermore, this protective effect of the blockade of complex 1-mtROS with OP2113 was also confirmed by restoration of the ATP production. By suppressing this formation of toxic reactive oxygen species, this compound has the advantage over reactive oxygen scavengers, in that it prevents tissue damage at a much earlier stage than just “mopping up” the reactive oxygen species after they have already been released. Whether OP2113 and its active substance ATT could consistently reduce myocardial infarction and/or no-reflow phenomenon [21] in a standardized rat model that focused on anatomic size of the risk zone, the infarct size, and the no-reflow size was not previously known.
Our present observation that OP2113 was able to reduce myocardial infarction (22% decrease in infarct size) is important as even 5% reductions in infarct size translate to improvements in heart function and clinical outcomes [22]. However, it does appear that OP2113 would need to be on board very early during the course of the myocardial infarction to show benefit. Therefore, prophylactic administration of the agent in high-risk patients might be one approach. However, there was even a trend for a reduction in infarct size with therapy prior to reperfusion, but larger numbers of experiments or higher doses of OP2113 would need to be done to positively confirm this observation. The fact that OP2113 reduced the size of the no-reflow zone, whether given in either a preventive or curative fashion, was an unexpected finding. Agents that reduce no reflow are sometimes coupled to their ability to reduce myocardial infarct size. However, we have observed that some therapies, such as late administration of therapeutic hypothermia, are capable of reducing the size of the no-reflow area, independent of any effect on infarct size [23]. Clinical studies have now shown that no-reflow is an independent risk factor (independent of myocardial infarct size) for poor clinical outcome including mortality and heart failure [24]. The presence of no-reflow zone is associated with adverse LV remodeling. This makes sense, since if there is a large area of no-reflow, the necrotic debris left after an acute myocardial infarction will have a harder time being removed from the zone of infarction, and healing cytokines and cells that help clean up the necrotic debris and contribute to the healing phase of infarction will have a harder time entering the necrotic zone due to a lack of blood perfusion into the ischemic risk area [24]. Whether OP2113 is capable of improving the healing phase of the left ventricle and reducing adverse LV remodeling after infarction is unknown at this time but hopefully will be studied in the future (Figs. 3, 4, and 5).
Representative images of the ischemic risk area, infarct area, and no-reflow area of the heart. Panels a, d, and g show the ischemic risk area. The blue area represents the blood perfusion region (non-ischemic area) and the pink area represents the region that does not receive blood perfusion (ischemic risk area) during left coronary artery occlusion. Panels b, e, and h show the no-reflow area. Under UV light, the fluorescent perfusion defect area (no-reflow area, which did not receive thioflavin S perfusion) is visualized as the dark area (red arrows). Panels c, f, and i show the infarct area. After incubation in TTC, viable tissue is stained with brick red, and necrosis or infarct tissue appears as a homogeneous white area (black arrows). Panels (a, b), and (c) from the same heart slice in the control group; (d, e), and (f) from the same heart slice in the curative group; and (g), (h), and (i) from the same heart slice in the preventive group (scale bar = 0.5 cm)
(a) The infarct size, which was expressed as percentage of the left ventricle ischemic risk area, was significantly smaller in the preventive group compared with the control saline group (p < 0.05). (b) The association between the myocardial ischemia risk zone and extent of necrosis in the 3 groups. As expected, the larger the ischemic risk zone as a percentage of the left ventricle, the larger the infarct size as a percentage of the left ventricle. There is a downward shift of the regression lines in the preventive group compared with the control group, which indicated that, for the same size of the risk area, the infarct size was smaller in the preventive group compared with the control group
(a) The no-reflow size, which was expressed as percentage of the left ventricle ischemic risk area, was significantly smaller in both curative and preventive groups compared with the control saline group. Control group versus curative group p = 0.043; control group versus preventive group p = 0.026. (b) The association between the myocardial ischemia risk zone and extent of no-reflow zone in the 3 groups. There is a downward shift in regression lines for the 2 treatment groups
There have been many studies that have tried to reduce myocardial infarct size and no reflow using mitochondrial protective approaches. Some have involved targeted mitochondrial bioenergetics, reactive oxygen species, inhibition of mitochondrial respiration, post-translational modifications, mitochondrial membrane potential, the mitochondrial permeability transition pore, and a host of older studies aimed at opening the mitochondrial K-ATP channel [15]. While some interventions showed promise, others showed mixed results, and none has shown promising effects in clinical trials. New mitochondrial peptides, such as elamipretide, which is thought to stabilize cardiolipin and maintain the respirasomes, had been shown to reduce myocardial infarct size in some but not all preclinical studies [25], while the peptide SBT-20 showed promise for reducing infarct size [26]. However, elamipretide did not reduce myocardial infarct size in a sizable clinical trial [27]. Preclinical and a small clinical trial showed promise that the mitochondrial permeability transitions pore inhibitor, cyclosporine A, could reduce infarct size [28]. However, large clinical trials with this agent were disappointing [29]. The reasons for negative clinical trials were discussed in detail by Botker et al. [30] and may include comorbidities including age, sex, other cardiovascular risk factors, and co-medicines that could suppress or mask a benefit. In addition, some of the preclinical studies that were performed did not always show reproducible results. The present study investigates the mitochondrial protective agent OP2113. In our study, which included a robust number of experiments, the drug showed promise, especially if it was on board early. Other studies in isolated global ischemia models in rodents, in vitro studies utilizing human cardiomyocytes, and myocardial infarct models in sheep have also shown promising results. This agent would be of interest to test in a clinical trial of acute ST elevation myocardial infarction, examining its effect on both myocardial infarction and no reflow (microvascular obstruction). However, because numerous adjunctive therapies have been developed to be administered along with reperfusion, but only a handful have shown promise in clinical trials [31], OP2113 may fail to show consistent protection in a clinically relevant intervention.
The ketamine and xylazine combination is a commonly used injectable anesthetic in rats for cardiovascular function assessment. Ketamine and xylazine can affect cardiovascular function in a dose-related manner [32, 33]. Picollo et al. [34] reported that rats were anesthetized with intraperitoneal injection of ketamine/xylazine and found that ketamine and xylazine mixture induced hypotension at 10 min after administration, and the hypotension was more prominent at 150 min. Afshar et al. [35] also reported the ketamine/xylazine combination was responsible for declined arterial blood pressure and bradycardia in anesthetized goats. Blood pressure was low throughout the experiments in all the groups in our present study. We suggested that the hypotension is caused by ketamine and xylazine in a dose-related manner, because rat blood pressure is back to normal now in our lab after we adjust the dose of ketamine and xylazine. Whether low blood pressures could provoke myocardial injury remains unclear. Walsh et al. [14] reported that even short durations of an intraoperative MAP less than 55 mmHg are associated with myocardial injury in patients. However, in our present study, MAP less than 55 mmHg did not worsen the ischemic/reperfusion-induced myocardial injury compared with MAP greater than 55 mmHg. The explanation may be due to the coronary blood flow that can keep nearly constant as pressure is reduced from 100 to 40 mmHg through coronary pressure-flow autoregulation [36, 37]. Moreover, the minimum coronary perfusion pressure needed for maintaining viable coronary blood flow is 20 mmHg [38].
Conclusions
In summary, our findings indicate that cells treated with OP2113 showed differences in succinate-induced mtROS generation, mitochondrial oxygen kinetics, and energy homeostasis. The changes observed in these three parameters are consistent with a protective effect of OP2113 in the situation of I/R and succinate accumulation that trigger excessive mtROS generation. Furthermore, OP2113 significantly reduced no-reflow area in both the curative and preventive groups compared with the control group and significantly reduced the infarct size in the preventive group compared with the control group. OP2113 treatment did not influence heart rate and arterial blood pressure during the procedure among the 3 groups.
References
Han H, Wei X, He Q, Yu Y, Ruan Y, Wu C, et al. Comparison of in-hospital mortality and length of stay in acute ST-segment-elevation myocardial infarction among urban teaching hospitals in China and the United States. J Am Heart Assoc. 2019;8(22):e012054.
Bahit MC, Ajar Kochar A, Granger CB. Post-myocardial infarction heart failure. JACC Heart Fail. 2018;6(3):179–86.
Detaille D, Pasdois P, Sémont A, Santos PD, Diolez P. An old medicine as a new drug to prevent mitochondrial complex I from producing oxygen radicals. PLoS One. 2019;14(5):e0216385.
Amoedo ND, Dard L, Sarlak S, Mahfouf W, Blanchard W, Rousseau B, et al. Targeting human lung adenocarcinoma with a suppressor of mitochondrial superoxide production. Antioxid Redox Signal. 2020. https://doi.org/10.1089/ars.2019.7892 Online ahead of print.
Sloan RC, Moukdar F, Frasier CR, Patel HD, Bostian PA, Lust RM, et al. Mitochondrial permeability transition in the diabetic heart: contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. J Mol Cell Cardiol. 2012;52(5):1009–18.
Gnaiger E. Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 4th ed. Innsbruck: Oroboros MiPNet Publications; 2014. www.bioblast.at/index.php/Gnaiger_2014_MitoPathways
Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955;217:383–93.
Makrecka-Kuka M, Krumschnabel G, Gnaiger E. High-resolution respirometry for simultaneous measurement of oxygen and hydrogen peroxide fluxes in permeabilized cells, tissue homogenate and isolated mitochondria. Biomolecules. 2015;5:1319–38.
Alleman RJ, Tsang AM, Ryan TE, Patteson DJ, McClung JM, Spangenburg EE, et al. Exercise-induced protection against reperfusion arrhythmia involves stabilization of mitochondrial energetics. Am J Physiol Heart Circ Physiol. 2016;310(10):H1360–70.
Gnaiger E, Steinlechner-Maran R, Méndez G, Eberl T, Margreiter R. Control of mitochondrial and cellular respiration by oxygen. J Bioenerg Biomembr. 1995;27(6):583–96.
Li Y, Kloner RA. Is there a gender difference in infarct size and arrhythmias following experimental coronary occlusion and reperfusion? J Thromb Thrombolysis. 1995;2(3):221–5.
Dow JS, Bhandari A, Hale SL, Kloner RA. Does sex influence the incidence or severity of reperfusion-induced cardiac arrhythmias? Springerplus. 2015;4:96.
Gnaiger E. Oxygen conformance of cellular respiration. A perspective of mitochondrial physiology. Adv Exp Med Biol. 2003;543:39–55.
Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507–15.
Kloner RA, Brown DA, Csete M, Dai W, Downey JM, Gottlieb RA, et al. New and revisited approaches to preserving the reperfused myocardium. Nat Rev Cardiol. 2017;14(11):679–93.
Kloner RA. Mitochondrial protective agents for ischemia/reperfusion injury. Circ Cardiovasc Interv. 2017;10(9):e005805.
Lesnefsky EJ, Chen Q, Tandler B, Hoppel CL. Mitochondrial dysfunction and myocardial ischemia-reperfusion: implications for novel therapies. Annu Rev Pharmacol Toxicol. 2017 Jan 6;57:535–65.
Dröse S, Stepanova A, Galkin A. Ischemic A/D transition of mitochondrial complex I and its role in ROS generation. Biochim Biophys Acta. 2016;1857(7):946–57.
Murphy MP, Hartley RC. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov. 2018;17(12):865–86.
Prag HA, Gruszczyk AV, Huang MM, Beach TE, Young T, Tronci L, Nikitopoulou E, Mulvey JF, Ascione R, Hadjihambi A, Shattock MJ, Pellerin L, Saeb-Parsy K, Frezza C, James AM, Krieg T, Murphy MP, Aksentijević D. Mechanism of succinate efflux upon reperfusion of the ischemic heart. Cardiovasc Res. 2020;cvaa148. https://doi.org/10.1093/cvr/cvaa148. Online ahead of print.
Kloner RA, King KS, Harrington MG. No-reflow phenomenon in the heart and brain. Am J Physiol Heart Circ Physiol. 2018;315(3):H550–62.
Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, et al. Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J Am Coll Cardiol. 2016;67(14):1674–83.
Kloner RA. No-reflow phenomenon. A new target for therapy of acute myocardial infarction independent of myocardial infarct size. J Cardiovasc Pharmacol Ther. 2018;23(3):273–6.
Kloner RA. No-reflow phenomenon: maintaining vascular integrity. J Cardiovasc Pharmacol Ther. 2011;16(3–4):244–50.
Kloner RA, Hale SL, Dai W, et al. Reduction of ischemia/reperfusion injury with bendavia, a mitochondria-targeting cytoprotective peptide. J Am Heart Assoc. 2012;1(3):e001644.
Dai W, Cheung E, Alleman RJ, Perry JB, Allen ME, Brown DA, et al. Cardioprotective effects of mitochondria-targeted peptide SBT-20 in two different models of rat ischemia/reperfusion. Cardiovasc Drugs Ther. 2016;30:559–66.
Gibson CM, Giugliano RP, Kloner RA, Bode C, Tendera M, Jánosi A, et al. EMBRACE STEMI study: a phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous elamipretide (MTP-131) on reperfusion injury in patients undergoing primary percutaneous intervention. Eur Heart J. 2016;37(16):1296–303.
Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359(5):473–81.
Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373(11):1021–31.
Bøtker HE, Cabrera-Fuentes HA, Ruiz-Meana M, Heusch G, Ovize M. Translational issues for mitoprotective agents as adjunct to reperfusion therapy in patients with ST-segment elevation myocardial infarction. J Cell Mol Med. 2020;24(5):2717–29.
Kloner RA. Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ Res. 2013;113(4):451–63.
Xu Q, Ming Z, Dart AM, Du XJ. Optimizing dosage of ketamine and xylazine in murine echocardiography. Clin Exp Pharmacol Physiol. 2007;34(5–6):499–507.
Clanachan AS, McGrath JC. Effects of ketamine on the peripheral autonomic nervous system of the rat. Br J Pharmacol. 1976;58(2):247–52.
Picollo C, Serra AJ, Levy RF, Antonio EL, dos Santos L, Tucci PJF. Hemodynamic and thermoregulatory effects of xylazine-ketamine mixture persist even after the anesthetic stage in rats. Arq Bras Med Vet Zootec. 2012;64(4):860–4.
Afshar FS, Baniadam A, Marashipour SP. Effect of xylazine-ketamine on arterial blood pressure, arterial blood pH, blood gases, rectal temperature, heart and respiratory rates in goats. Bull Vet Inst Pulawy. 2005;49:481–4.
Canty JM Jr, Smith TP Jr. Modulation of coronary autoregulatory responses by endothelium-derived nitric oxide. Int J Cardiol. 1995;50(3):207–15.
Goodwill AG, Dick GM, Kiel AM, Tune JD. Regulation of coronary blood flow. Compr Physiol. 2017;7(2):321–82.
von Planta I, Weil MH, von Planta M, Bisera J, Bruno S, Gazmuri RJ, et al. Cardiopulmonary resuscitation in the rat. J Appl Physiol (1985). 1988;65(6):2641–7.
Funding
Dr. Robert A. Kloner is the principle investigator of this work, and the project was supported by OP2 Drugs, Pessac, France.
Author information
Authors and Affiliations
Contributions
The authors made substantial contributions to the conception or design of the work (R.A.K.) or to the acquisition, analysis, or interpretation of data for the work (W.D., N.D.A., J.P., B.L.G., A.B., J.C., L.Z., D.A.B., R.R., R.A.K.); participated in critically revising the manuscript (W.D., N.D.A., J.P., B.L.G., A.B., J.C., L.Z., D.A.B., R.R., R.A.K.); approved the final version to be published (W.D., N.D.A., J.P., B.L.G., A.B., J.C., L.Z., D.A.B., R.R., R.A.K.); and agreed to be accountable for all aspects of the work (W.D., N.D.A., J.P., B.L.G., A.B., J.C., L.Z., D.A.B., R.R., R.A.K.).
Corresponding author
Ethics declarations
Conflict of Interest
Aurélie Boucard and Bruno Le Grand are employees for OP2 Drugs. Rodrigue Rossignol is a scientific advisor for OP2 Drugs. There are no conflicts of interest for Drs. Dai, Amoedo, Perry, Carreno, Zhao, Brown, and Kloner.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dai, W., Amoedo, N.D., Perry, J. et al. Effects of OP2113 on Myocardial Infarct Size and No Reflow in a Rat Myocardial Ischemia/Reperfusion Model. Cardiovasc Drugs Ther 36, 217–227 (2022). https://doi.org/10.1007/s10557-020-07113-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-07113-7