Abstract
Increasing evidence suggests that there are significant differences in the presentation, diagnosis and treatment of ischemic heart disease in women compared to men. Women often present with atypical symptoms, and this, in association with a consistent underestimation of their risk for ischemic heart disease, leads to underdiagnosis and undertreatment in women. Cardiovascular risk factors unique to women have only recently been recognized, and moreover, traditional risk factors have recently been shown to have greater impacts on women. Consequently, women suffer more disability and poorer clinical outcomes, with higher cardiovascular morbidity and mortality. These discrepancies may in part be secondary to the higher prevalence of nonobstructive coronary artery disease in women with persistent chest pain symptoms as compared to men when evaluated invasively. Focused diagnostic and therapeutic strategies unique to women are thus needed, but unfortunately, such sex-specific guidelines do not yet exist, largely due to lack of awareness, both on the part of providers and patients, as well as a paucity of evidence-based research specific to women. Although underutilized in women, diagnostic modalities, including functional and anatomic cardiac tests as well as physiologic assessments of endothelial and microvascular function, are useful for establishing the diagnosis and prognosis of suspected ischemic heart disease in women. This review discusses the current challenges of prevention, diagnosis and treatment of ischemic heart disease in women.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Current State of Ischemic Heart Disease in Women
The number of men and women who are affected by and die from coronary artery disease (CAD) outnumber all other conditions including all forms of cancer in the US [1]. However, there are distinct differences in the experience of CAD among women in comparison to men. Several studies have demonstrated perplexing diagnostic and management dilemmas in women due to their lower prevalence of angiographically obstructive CAD, greater symptom burden and rate of functional disability in comparison to their male counterparts [2]. These discrepancies have called for a more inclusive term, “ischemic heart disease” (IHD), in women to capture a wider spectrum and definition of a sex-specific pattern of CAD in women [3]. There is consistent evidence that adverse outcomes in women with IHD may be fueled by underestimation of cardiovascular disease (CVD) risk, leading to underdiagnosis and undertreatment. The reasons for these gender disparities are uncertain; therefore it is crucial to elucidate the interplay of key clinical, pathophysiological and psychosocial determinants in the evolution of IHD. It is essential that a focus be placed on the primary and secondary prevention of CVD in women to not only alleviate the exuberant economic burden of associated health care costs, but to also to reduce its associated effects on mortality and well-being.
Most of our knowledge and guidelines directing the prevention, management and treatment of IHD and its risk factors are based on data from randomized clinical trials with small proportions of women participants. This underrepresentation was confirmed by an assessment of females in clinical trials from 1997 to 2006 which was estimated at only 27 % [4]. Furthermore, the substantial heterogeneity across studies and lack of consideration of sex-specific factors in study design and implementation, limit the ability to draw more conclusive inferences [5]. In addition, there are a disproportionately small number of studies addressing CVD in women, but studies have instead overwhelmingly targeted reproductive concerns, termed “bikini medicine” [6]. As such, there remains uncertainty in women-specific clinical manifestations and management of IHD as many algorithms that we use today are derived from predominantly male populations.
This review outlines the current challenges in the primary and secondary prevention, diagnosis and management of IHD in women. We present a comprehensive selection of key evidence highlighting the epidemiology, risk factors, screening, diagnosis and treatment of IHD in women. We identify gaps in knowledge of IHD in women which in turn may spur further sex-specific studies and interventions towards the improvement of cardiac care and outcomes in women.
Epidemiology of Ischemic Heart Disease
Incidence and Prevalence
The view of CAD as a “man’s disease” is slowly dissipating as it has emerged as a major cause of morbidity and mortality amongst women. Among Americans aged 20 years or older, 15.4 million have CAD with 5 % of these individuals being women [1]. Black women have a higher prevalence of 7 % compared to 4.6 % among white women. Overall, the prevalence of CAD is lower in middle-aged women than in men according to the most recent iteration for the National Heart, Lung, and Blood Institute (NHLBI), National Health and Nutrition Examination Survey (NHANES); however there exists an overall upward trend in women [7], especially younger women. This data is likely an underestimation as it only accounts for obstructive CAD (angiographically-determined stenosis >50 %) and does not include other forms of IHD.
As women age, the incidence of all initial coronary events including myocardial infarction (MI), angina pectoris, unstable coronary syndromes and coronary deaths) increases and eventually approaches that of men by age 60 [8–10]. There is a lag time period of about 10 years in the incidence of all coronary events in women behind men which increases to about 20 years for critical events such as MI and sudden death [1]. Notably, the incidence of total coronary events triples in women over age 65 compared to younger women [11]. There is evidence of a racial disparity as black women aged 45 to 64 within the Atherosclerosis Risk in Communities (ARIC) study were significantly more likely than their white counterparts to experience CVD death as a first event [12]. Discouragingly, recent statistics indicate that although the overall CVD mortality is decreasing for both men and women, it is accelerating in younger women, especially those in mid-life [13, 14].
Clinical Presentation
The clinical assessment of women with IHD has been traditionally viewed through the lens of “typical” angina symptoms characteristic of primarily male study cohorts. Interestingly, effort angina is of similar or increased prevalence among women in comparison to their male counterparts [15, 16]. Yet, a wide range of “atypical” symptoms occur more frequently in women including nausea, fatigue, dyspnea, weakness as well as unconventional descriptors, triggers and locations of chest-related symptoms [17, 18]. Some have suggested that lack of existence of a female-specific characterization of IHD symptoms has resulted in suboptimal care and outcomes among women as an emphasis has been placed on identifying noncardiac etiologies to chest pain that is not “typical.” [17] Of clinical relevance is the fact that the presence of symptoms alone, whether “typical” or “atypical” places women at a greater risk of future cardiovascular events [19]. Black and white women differ in their symptom presentation and this difference is associated with a worse prognosis among black women [18]. Strikingly, women are more likely to not report anginal symptomatology as it seems as if a disconnect exists between perception of symptoms and health status [20].
Obstructive versus Nonobstructive CAD
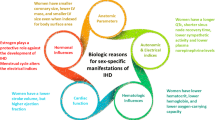
Despite having more symptomatology and debility than men, women have less anatomical obstructive CAD [21, 22]. Several studies have confirmed the clinical observation that women have a lower plaque burden than men, including atheroma within the media and luminal plaque [21]. In an effort to tackle this issue of clinicopathophysiological differences of IHD in women, the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study sought to better elucidate the complexity of IHD in women. Of over 800 women in the cohort who underwent clinically-indicated angiograms, 62 % were not found to have obstructive CAD at catheterization [23]. These findings were further corroborated within the American College of Cardiology (ACC)-National Cardiovascular Data Registry (NCDR), as 51 % of women with stable angina referred for coronary angiography had nonobstructive disease compared with 32 % of men [24]. Approximately two-thirds of the black women studied within the Coronary Artery Surgery Study (CASS) registry had nonobstructive CAD in comparison to slightly over half of their white counterparts [25]. The issue remains whether women experience myocardial ischemia by a different pathophysiology than men, as they more commonly do not have obstructive CAD. The lack of “significant stenosis” approach to management has been a serious detriment to women as the absence of demonstrable obstructive CAD in women with persistent IHD symptoms is not benign [15, 26].
Cardiovascular Mortality
Positively speaking, the US mortality rate from CVD in men and women has had a 39 % decline over the past decade [1]. However, the leading cause of death among women remains CAD. Despite innovations in cardiac medical therapies and care, greater than 250,000 women in the US die annually from CAD-related deaths-five-fold higher than women with breast cancer [1, 2, 27]. There is an even greater disparity among middle-aged black women as they have a 2.5 times higher mortality from CAD than similarly-aged white women [2, 27, 28]. It is also striking that women are more likely to die after their first MI whereas men have four times more coronary events than women [1]. Nevertheless, nearly half of all American women, especially those younger than 50 and/or of ethnic diversity, remain unaware that IHD is their greatest health threat [29].
There is equipoise in the current evidence regarding mortality rates in women after an acute coronary event with some studies revealing higher death rates or even a survival advantage in women [5, 30]. The longer term outcomes are even more inconclusive [5]. However, the vast majority of studies have reported higher mortality rates for women compared with men after an acute MI [5], but this trend may be explained by age, higher prevalence of cardiac risk factors, poorer clinical presentation and treatment differences. Older age and increased comorbidities at presentation such as diabetes, hypertension and heart failure, may further clarify this differential. There is also evidence suggesting worse mortality rates in younger women following an acute MI [5, 31–35]. Fortunately, differences in mortality risk following percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) appear to be narrowing between men and women [5, 35]. This has been attributed to advances in revascularization techniques and therapies and improved guideline adherence.
A puzzling paradox exists when examining cardiac event and mortality rates among women with nonobstructive CAD. Among the WISE cohort, there is a trend towards increased fatal and nonfatal cardiovascular event rates (MI, stroke, and congestive heart failure) with age, with the major difference emerging after age 54 [19]. Most salient is the fact that the risk of cardiac events for symptomatic women with nonobstructive CAD is almost double that of symptomatic women with normal coronaries. Correspondingly, among women without CAD, those with persistent chest pain in spite of medical therapy had twice the rate of future cardiac events than asymptomatic women [36].
Healthcare Cost Burden
CVD constitutes 17 % of the national health expenditures, with the annual direct and indirect costs of care for women at an estimated $130 billion [1, 28]. Much of these exuberant healthcare costs are associated with the diagnosis and management of persistent angina in women without obstructive CAD. An annual excess expenditure of $280 million has resulted from the over half a million coronary angiograms completed in women which in only half of the cases are revealing of actual flow-limiting stenoses [37]. This estimate does not account for the incurred continued longitudinal medical assessments including increased office visits, procedures and hospitalizations for women with persistent chest pain [37]. The average lifetime cost estimate is approximately $770,000 and ranged from $1.0 to $1.1 million for women with nonobstructive CAD which approaches that of women with obstructive CAD [23]. This presents an enormous challenge to clinicians in treating these women with a greater symptom burden but no evidence of the classically described male pattern of obstructive CAD (>50 % stenosis).
Quality of Life
Persistently symptomatic women with IHD require more hospitalizations and repeat invasive procedures in comparison to men which undoubtedly lead not only to increased health care costs but more importantly, lower ratings of quality of life, general well-being and productivity among women. Despite similar lifestyle and pharmacologic management strategies, women with angina have been shown to have inferior functional status scores than men even after adjustment for confounders such as CAD severity and comorbid conditions [20]. Women with IHD are likely to have higher rates of depression, anxiety and inadequate social support which may have a detrimental effect on physical functioning [20, 38–40]. Clearly, the implications of this disparity in psychosocial well-being are substantial and deserve further attention in the clinical care of women with IHD.
Risk Factors for Ischemic Heart Disease
Traditional Risk Factors
Traditional risk factors including family history of premature CAD, age, smoking, hypertension, diabetes, dyslipidemia, obesity and physical inactivity are well-documented in the etiological IHD pathway in women. Over 80 % of middle-aged women have ≥1 traditional cardiac risk factor [3]. The majority of risk factors for black women are attributed to diabetes, hypertension, overweight/obesity, and physical inactivity, as compared to white women who proportionally have more smoking and hypercholesterolemia [1].
Unfortunately, most “traditional” CVD risk factors are associated with proportionally greater risk in women. Female relatives with premature CAD confer a more potent risk to family members than male relatives with premature CAD [41]. Hypertension is a major risk factor in women which becomes more prevalent with age and is particularly prevalent in black women [1]. Diabetic women have a 3-fold higher risk for CAD in comparison to nondiabetic women and have significantly greater IHD mortality rate than diabetic men. Lipid profiles in women deteriorate in the perimenopausal and post-menopausal phases of life, with reductions in “good” (HDL) and increases in “bad” (LDL) cholesterol; indeed women develop higher cholesterol levels than men after the fifth decade of life [42–44]. Higher triglyceride levels are a more prevalent and potent, independent risk factor for IHD in women than in men [45–47]. Moreover, smoking has been identified as a stronger risk factor for IHD among middle-aged women in comparison to men [48, 49], conferring approximately twice the risk in women.
The so-called “graying” or aging of America projected for 2020 and beyond will undoubtedly influence patterns in CVD epidemiology and healthcare costs, particularly for women. Women experience a more exponential increase in IHD after age 60, whereas men have a more linear increase [50]. Despite the clear evidence that both men and women with optimal risk factor profiles have lower risks of IHD compared to those with suboptimal profiles, less than 2 % of the US population in NHANES (75 % women) actually met the seven simple ideal cardiovascular health metrics [51, 52]. Although women are increasingly aware of CVD as the “number one killer of women” there remain significant disconnects between this awareness and perceived individual risk [53] which is especially significant for women who are younger and of diverse ethnicity [29].
Unique and Emerging Risk Factors
There are several newly-identified cardiac risk factors for women. The examination of those unique to, or more common in, women may offer insight into the tailoring of current risk assessment algorithms for women. Metabolic syndrome has emerged as a clustering of cardiometabolic risk factors [glucose intolerance, central obesity, hypertension, dyslipidemia (low HDL, high triglycerides)] and is more common after menopause [26]. Thus, it is often linked with hormonal alterations [26, 54] and is associated with a markedly higher risk of IHD and cardiac events. Furthermore, high-sensitivity C-reactive protein (hsCRP) may improve risk stratification for IHD in women, particularly those with metabolic syndrome [55, 56]. High-sensitivity C-reactive protein has consistently been higher in women than in men after puberty and there is clear variation with estrogen levels in postmenopausal women [57]. Recent evidence has emerged suggesting a connection between autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, which are more common in women, and increased risk of IHD [58].
Hormonal fluxes over a woman’s lifespan also influence IHD risk, and provide unique risk factors, seen only in women. It has been observed that early menarche (<12 years at onset) increases subsequent risk of cardiac events and both CVD and overall mortality [59]. Entities causing ovarian dysfunction, such as functional hypothalamic amenorrhea, have been associated with premature coronary atherosclerosis and associated CVD events [60]. Moreover, polycystic ovarian syndrome (PCOS) is coupled with risk factor clustering including diabetes, obesity and the metabolic syndrome, thus leading to heightened IHD risk [3]. The recent effectiveness-based prevention guidelines for women have identified pre-eclampsia and gestational diabetes as “at risk” categories for IHD [61] and there is further supportive evidence linking these entities to a 2-fold increased CVD risk [62].
Microvascular and Endothelial Dysfunction
The astonishing prevalence of “normal” or “near-normal” epicardial arteries in women with chest pain, suggests alternative pathophysiological mechanisms from the classic demand-supply mismatch of flow-limiting coronary artery stenosis. Possible explanations for this chest pain syndrome, often termed “nonobstructive CAD”, include abnormal coronary reactivity, plaque erosion/distal microembolization and microvascular or endothelial dysfunction as contributory to a female-specific IHD pattern [21]. These mechanisms are characterized by impairment in vasomotor tone and vascular homeostasis which lead to characteristic ischemic symptoms [20, 63]. Close to one half of the women presenting with chest pain in the presence of nonobstructive CAD within the WISE study had coronary microvascular dysfunction as determined by invasive [64] and noninvasive methods such as magnetic resonance imaging [65, 66]. Further evidence suggests the clinical and prognostic importance of impaired coronary vasomotion, as its detection was associated with adverse cardiovascular outcomes irrespective of CAD severity in the same cohort of women [67].
Traditional cardiovascular risk factors of increased prevalence and impact in women have been implicated in the development of endothelial dysfunction [20]. These conditions, whether alone or in conglomerate, lead to vascular endothelial injury and increased oxidative stress which further promote coronary atherogenesis [9]. Investigators have theorized that the higher prevalence of left ventricular hypertrophy and obesity in black women results in “microvascular angina” from decreased coronary vascular reserve [68]. There is also evidence of a higher risk of progression to atherosclerotic CAD in patients with endothelial dysfunction [69].
Risk Assessment for Prevention
Given the alarmingly high burden of cardiac risk factors in our population, there has been a timely shift toward primary and secondary prevention of IHD through enhanced risk stratification and assessment. Thus, the notion is to significantly reduce the prevalence of risk factors through therapeutic and lifestyle intervention with an anticipated alleviation of CVD events and mortality [28]. In terms of primary prevention, the classic Framingham risk score (FRS) has historically been the most prominent and widely used tool for estimating 10-year cardiovascular risk; however it has inherent limitations of underestimating risk in women. In women who sustained their first MI, the majority were classified in the low risk category by FRS score (95 %), with the remaining in the intermediate category (5 %) [70, 71]. Given the FRS shortcomings, a number of other global risk score calculators have debuted from different study cohorts including SCORE [72], QRISK [73]), the 2001 ATP-III Risk estimator (FRS-based) [74] in addition to the Reynold’s risk score. Ideally, scoring systems have the highest accuracy in the population from which they were developed [71]. This presents substantial room for inaccuracies in women and ethnic groups whom are disproportionately understudied. The Reynold’s risk score, which includes hsCRP, was derived from and validated in women cohorts and in comparison with the FRS resulted in improved risk prediction with reclassification in 15 % of intermediate-risk FRS women to high risk [75, 76].
The unveiling in 2013 of the new guidelines on treatment of cholesterol to reduce atherosclerotic cardiovascular risk (ASCVD) by the ACC and American Heart Association (AHA) generated much controversy though its aim was to avail to clinicians a more straightforward, evidence-based tool [77]. The vanguard instrument eliminates the use of a target cholesterol level, recommends a fixed statin intensity based on classified risk group, includes stroke as an endpoint and allows for estimates by sex and race. The guideline’s pooled cohort equation was originated and validated in men and women within geographically and racially representative populations including blacks [78]. Critics suggest that the novel score calculator overestimates risk by 75 to 150 % in at least seven external validation cohorts which could lead to excessive statin therapy [79, 80]. There remains disagreement among polarized academicians regarding the performance of the pooled cohort equation and conventional scoring systems. Nevertheless, with certainty, the outstanding issue remains- intermediate to high risk groups, including women, are in dire need of lifestyle and risk factor optimization for CVD risk reduction, and refined IHD detection to ideally prevent, or treat adverse CVD events.
Diagnosis of Ischemic Heart Disease
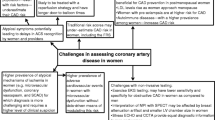
The diagnosis of IHD in women is more challenging and is frequently delayed as women commonly present with delayed onset of frequently atypical symptoms. Women are usually evaluated for CAD about 10-20 years later than men. Although the majority of women present with the same symptoms of CAD as men, a significant number also experience atypical symptoms. For example, in a large study of patients diagnosed with myocardial infarction, 58 % of women compared to 69 % of men were reported to describe chest pain as their presenting symptom [81]. Moreover, when women with acute coronary syndromes (ACS) undergo cardiac catheterization, at least twice as many women as compared to men, will have no significant obstructive CAD, yet their prognosis is worse than that of both men and women who do not have chest pain syndromes [36]. This makes the diagnosis of IHD in women more challenging. Most often, those individuals with other than the characteristic “male” pattern of obstructive CAD at coronary angiography, are simply reassured, and not offered additional testing or treatments, nor guidance on reduction of ASCVD risk. Even more complex are those women who present with ACS that represent manifestations of coronary disease that are very poorly understood, but far more common in women, including stress-induced (Takotsubo, left ventricular apical ballooning) cardiomyopathy, spontaneous coronary artery dissection, coronary vasospasm and coronary embolism. These entities once thought “rare” are increasingly being diagnosed in women. Additional imaging techniques, including MRI with late gadolinium enhancement [82] echocardiography with ultrasound enhanced cardiac perfusion [83] intravascular ultrasound and optical coherence tomography [84, 85] are assisting in establishing the diagnosis and pathophysiologic understanding of these less common acute cardiovascular entities, in order to determine and guide the most appropriate therapy.
Unfortunately, current guidelines on the management of acute and stable cardiac ischemic syndromes do not include a sex-based diagnostic approach. It is important to underscore that the majority of available multicenter clinical studies and trials used to support current guidelines are based on predominantly male populations. Within these limitations, we will review the current noninvasive and invasive approaches to the diagnosis of IHD in women, including functional testing (stress testing), anatomic imaging (coronary computed tomography (CT), and endothelial function assessments).
Noninvasive Testing
The 2014 AHA Consensus Statement on the “Role of Noninvasive Testing in the Clinical Evaluation of Women with Suspected Ischemic Heart Disease,” provides evidence-based guidelines on diagnosis of IHD in women by non-invasive testing [86]. The choices of non-invasive testing are similar between men and women. However, women are more likely to have “false positive” results, and due to a lack of confidence in accuracy, these non-invasive diagnostic tests are often improperly utilized [86]. Pretest probability must be taken into account when determining the need for ASCVD assessment. Initial pretest assessment for exercise capacity is important to ascertain whether a woman can exercise to an adequate level at which ischemia may develop. In women unable to perform activities of daily living or to perform adequately on exercise treadmill testing (ETT), a pharmacological stress test is the preferred method of risk assessment. Stress imaging tests provide information about wall motion abnormalities or perfusion, and provide assessment of ventricular function.
Functional Testing
Functional tests include ETT with electrocardiogram (ECG), exercise/pharmacologic stress echocardiography, exercise/pharmacologic cardiac nuclear imaging with single-photon emission computed tomography (SPECT) or positron emission tomography (PET), pharmacologic stress cardiac magnetic resonance imaging (CMR), CT perfusion and CT or Doppler ultrasound-derived flow reserve measurements.
ETT is the most common method of diagnosing CAD in women despite a higher false-positive rate compared to men. ETT is recommended as the diagnostic test of choice in symptomatic, intermediate risk women who are able to exercise and have an interpretable resting ECG. Exercise stress testing provides valuable information about exercise capacity, and hemodynamic response to exercise and recovery, all markers of cardiovascular risk. Women who are unable to exercise beyond stage 1 of a standard Bruce protocol, achieving <4-5 metabolic equivalents, are at the highest risk of cardiovascular events and this portends worse clinical outcome [87]. This is in contrary to women achieving exercise workloads of >10 metabolic equivalents which predicts a very low risk of inducible ischemia [88]. Lack of appropriate blood pressure and heart rate increase with exercise, or a drop of blood pressure with exertion, are concerning for IHD in both men and women [89]. Regardless of gender, high risk patients identified by ETT demonstrate symptom limited angina and marked ST segment changes of ≥2 mm or downsloping ST segments in multiple leads. This threshold is however less accurate for detection of ischemia in women. Lower sensitivity and specificity of ST-segment responses with exercise has been documented [90]. Exercise capacity is further reflected by the Duke Treadmill Score, calculated as exercise time – (5 × ST segment changes in mm) – (4 × angina index). This scoring tool not only identifies high risk patients for CAD, but also provides prognostic information [91]. However, ETT testing can be limited by both reduced specificity and sensitivity in both women and men, and is not interpretable if there are resting ECG abnormalities, or the patient is unable to exercise.
A frequent reason for performing ETT in women is the high negative predictive value. In order to explore whether myocardial perfusion imaging (MPI) with SPECT could provide incremental information for diagnosis in symptomatic women at low to intermediate pretest probability over ETT alone, the “What is the Optimal Method for Ischemia Evaluation in Women” (WOMEN) trial was performed [92]. Similar 2-year clinical outcomes were observed, with no difference in major adverse cardiac events (MACE) (<3 %). Overall, the cumulative diagnostic cost savings was 48 % for ETT compared with exercise MPI. Thus, for symptomatic women with low to intermediate risk who are capable of exercising, ETT is the recommended initial test of choice to provide diagnostic and prognostic information.
As previously noted, the prevalence of obstructive CAD in women is lower than in men. The pretest probabilities of CAD are lower in women, and more false positive results for stress imaging have been reported. In women, the accuracy of stress echocardiography and its diagnostic sensitivity and specificity in detecting CAD is higher compared to exercise ECG [93–95]. In comparison, exercise echocardiography has higher sensitivity in men [96]. Despite these differences, the prognostic value of exercise echocardiography is comparable between men and women [97]. Women with low-risk stress imaging findings, have <1 % risk of CAD. Women with moderate to severe wall motion or perfusion abnormalities are at higher risk, and may have annual CAD event rates as high as 5–10 % per year, depending on the vascular territory and the choice of stress imaging used [97, 98]. Additionally, reaching a workload of >6 metabolic equivalents during exercise echocardiography was associated with decreased risk of cardiac events and cardiac death in both men and women [97].
Challenges in interpretation of stress imaging tests in women are technique-dependent. Nuclear stress testing challenges can occur due to breast tissue artifacts and smaller hearts of females. The smaller LV size may not allow detection of small perfusion abnormalities. New techniques however are currently used to overcome the frequency of attenuation artifacts. Questions about radiation safety associated with radionuclide stress tests have been raised [99], and tests utilizing ionizing radiation are frequently avoided or used cautiously in young women due to increased lifetime risk of cancer.
Anatomic Testing
In the last decade, the evidence regarding the utility of cardiac CT has grown exponentially. Coronary computed tomographic angiography (CCTA) and coronary artery calcium (CAC) score provide additional tools for assessing diagnosis and prognosis of CAD. CCTA can risk-stratify patients with acute chest pain and intermediate likelihood of ACS. CCTA shows the extent of both calcified and non-calcified plaque, obstructive and nonobstructive atherosclerosis, with increasingly lower radiation exposure and improved image quality. Data from the “Coronary CT Angiography Evaluation for Clinical Outcomes” (CONFIRM) trial showed that the presence of multi-vessel CAD in women by CCTA predicted a 3–4 fold higher risk of death [100]. The “Rule Out Myocardial Infarction using Computer Assisted Tomography” trial (ROMICAT), comprised of 40 % women, demonstrated that half of patients with acute chest pain at low to intermediate likelihood of ACS had no CAD by CCTA, with very high negative predictive value [101]. Two-year follow up of the ROMICAT study cohort revealed that CCTA predicts MACE and has incremental prognostic value in patients with acute chest pain. The probability of MACE within 2 years increased in parallel with increased burden of coronary disease (plaque, stenosis, left ventricular wall motion abnormalities) [102]. The subsequent ROMICAT II trial sought to examine gender differences in outcomes and found that women undergoing CCTA compared to standard cardiac evaluation had fewer hospital admissions, shorter length of hospital stay and lower total radiation dose compared with men. Thus, CCTA is a viable alternative for women undergoing assessment of CAD. Assessment of CAC score and its prognostic value in both men and women is rapidly evolving. CAC increases with age and is more substantial in men [103]. Women tend to have a less severe burden of atherosclerosis, with very low prevalence in premenopausal women. CAC scoring was shown to have similar predictive value for arteriographic CAD in men and women. The sensitivity of CAC for detection of obstructive disease is >95 % in women, and specificity of the test is significantly higher in women compared to men [104]. Therefore, CAC scoring also adds value in assessment of CAD in women, with minimal radiation exposure.
The recent “Prospective Multicenter Imaging Study for Evaluation of Chest Pain” (PROMISE) trial comparing functional tests (ETT, stress echocardiography, MPI) to anatomic assessment (CCTA), which had excellent female representation (50 % women), showed no significant differences in outcomes by strategy used [105]. Several additional multicenter clinical trials are underway comparing the role of different noninvasive tests which will further help in the diagnostic and therapeutic decision-making in stable patients with suspected IHD. The “Randomized Evaluation of Patients with Stable Angina” (RESCUE) trial compares CCTA with SPECT MPI. The NHLBI-sponsored “International Study of Comparative Health Effectiveness and Invasive Approaches” (ISCHEMIA) trial plans to randomize patients with chronic IHD with moderate to severe ischemia on stress imaging to therapy with invasive angiography or medical management. These studies will further expand our understanding of the diagnosis and treatment of suspected IHD in both men and women.
Microvascular Testing
Coronary microvascular disease (MVD), defined as limited coronary flow reserve and/or coronary endothelial dysfunction are the presumed mechanisms of ischemia in women with persistent angina, variable evidence of ischemia on stress testing, and no evidence of obstructive CAD on angiography. MVD is characterized by a decrease in the size of epicardial vessels and microvasculature, increased arterial stiffness, increased fibrosis, altered remodeling, more diffuse atherosclerotic disease, and the presence of endothelial or smooth muscle dysfunction [106]. MVD portends a worse prognosis in women with an estimated 2.5 % annual MACE rate in women [107]. In the last few decades, non-invasive and invasive techniques have evolved to adequately assess coronary physiology.
Noninvasive techniques such as PET, CMR and transthoracic echocardiography Doppler allow for the assessment of myocardial blood flow and coronary flow reserve. Decreased flow reserve in women is associated with worse outcomes, with increased rate of cardiac death, stroke or heart failure [19, 108]. Early detection of endothelial dysfunction, measured by brachial artery flow-mediated vasodilation, has also been associated with a 1.3 to 4.4-fold increase in IHD in women [109]. Additional simpler noninvasive techniques have emerged, with specially-designed fingertip probes to measure the peripheral reactive hyperemia index (PRHI), a measure thought to reflect endothelial function [110] and has been shown to be significantly reduced in the setting of persistent chest pain syndromes associated with nonobstructive CAD in women [111].
PET and CMR are growing noninvasive modalities to detect sub-endocardial ischemia; the gold standard is an invasive coronary reactivity test. The WISE study highlighted the importance of MVD in women [64] and supported the use of invasive coronary vasomotor testing as a safe method for definitive diagnosis and assessment of prognosis in high risk women [107]. It is now well established that the prognosis is worse in women with MVD and should not be underestimated by clinicians [112].
Invasive Testing
In women and men with a high pretest probability of CAD, coronary angiography is the mainstay of diagnosis and permits catheter-based therapy when indicated. As outlined above, evidence from the NCDR and the WISE studies, indicate that over 50 % of women with chest pain referred for coronary angiography do not have significant (>50 % stenosis of any one major coronary artery) obstructive CAD [2]. In the absence of significant obstructive CAD, strong consideration of coronary physiologic testing should be done, to evaluate for MVD and endothelial dysfunction. Although noninvasive techniques, as described above, are evolving for this, the gold standard remains catheter-based [113]. Pharmacologic assessment of coronary blood flow and flow reserve by cardiac catheterization, permits evaluation of both endothelium-dependent (using acetylcholine) and non-endothelium dependent (using adenosine, nitroglycerin or ergot alkaloids) mechanisms. [114] Endothelial dysfunction is defined as lack of increase in coronary blood flow after administration of endothelium-dependent vasodilators such as acetylcholine. Endothelial dysfunction is also one of the earliest markers of atherosclerotic disease. Coronary flow reserve is defined as the ratio of augmented to baseline blood flow after intracoronary administration of a vasodilator (adenosine, dipyridamole or regadenoson); normally, the ratio is >2.0. Although coronary physiologic testing does have potential risks and limitations, the evaluation for coronary vascular dysregulation, either invasively, or noninvasively, is recommended in women with persistent chest pain syndromes without obstructive CAD for proper diagnosis and effective treatment.
Treatment of Ischemic Heart Disease
Although our emerging understanding of IHD in women points to a differing pathophysiology than men, the recommended treatment of CVD in women is similar to that of men, with respect to both primary and secondary prevention, and ACS. According to the current ACC/AHA guidelines for management of ACS, indications for non-invasive/invasive diagnostic procedures and the treatment strategies should be implemented similarly for both men and women [115] with the overarching goal to improve quality of life and outcomes. However, despite these recommendations and goals of care, women continue to be treated less aggressively than men, with less intensive use of evidence-based medical and procedural therapy, less enrollment in cardiac rehabilitation, and less intensive therapeutic lifestyle counseling [116–119]. In a large international prospective study of over 30,000 men and women (22.6 %) with stable CAD, it was found that although risk profiles of men and women differed substantially, their one-year outcomes were similar, although fewer women underwent revascularization [120]. Further research is needed to better understand gender determinants of outcome and devise strategies to minimize bias in the management and treatment of women.
Therapeutic Lifestyle Intervention
Lifestyle modification, risk factor control and overall CVD prevention is paramount in women. Lifestyle interventions include smoking cessation, regular moderate intensity physical activity, dietary counseling for a heart healthy diet, weight reduction and maintenance, and treatment of depression if indicated. Major risk factor interventions include optimization of blood pressure, lipids, and glycemic control, as well as weight management through appropriate lifestyle interventions and medical therapy.
Medical Anti-Ischemic Therapy
Anti-ischemic medical therapy including aspirin, the angiotensin converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB), beta blockers, aldosterone inhibitors and statins are frequently delayed in women due to delay in symptom presentation and are less intensively used, despite their beneficial effects. These treatment differences in gender are possibly attributed to lower prevalence of obstructive CAD in women. The Euro Heart Survey showed that women were significantly less likely to receive aspirin and statin for treatment of stable angina [118]. After hospital discharge for non-ST-elevation MI, women received about 3 % less aspirin and beta blockers and about 13 % less statin therapy compared to men [116]. These are concerning findings, considering that statins and ACEI were shown to improve endothelial dysfunction, which is so prevalent in women.
Aspirin is recommended as part of management of ACS in both men and women. Although it has been shown to be equally beneficial for secondary prevention in both genders [121], it is less consistently used for primary prevention of CVD in women. In regards to primary prevention, it has been shown that aspirin prevents stroke in women older than 45 years old, and prevents MI in those over age 65 years [122]. Reduction in platelet reactivity in women after intake of low dose aspirin is at least similar to that of men [123] and based on the results from the Women’s Health Study, the reduction of thromboxane and prostacyclin is also similar between men and women [124]. Recent clinical trials, including the “Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin” (JUPITER) trial [125], Heart Protection Study [126], “The Cholesterol and Recurrent Events” (CARE) trial [127] and “The Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22” (PROVE IT-TIMI 22) trial [128], focused on cholesterol-lowering in patients with CVD and demonstrated at least similar reduction in cardiovascular morbidity and mortality for both men and women.
Therapies for Acute Coronary Syndromes
According to the 2014 ACC/AHA guideline for management of ACS, it is recommended that women be treated in a similar manner to men with the same indications for noninvasive and invasive testing. Large scale observation from the CRUSADE initiative [116] showed that despite these recommendations, women are treated less aggressively, with less cardiac catheterizations, PCIs, fibrinolysis procedures or CABG, which may contribute to different clinical outcomes. Recent meta-analysis comparing early invasive versus conservative treatment in men and women with unstable angina [129] showed similar reductions of death, MI or recurrent ACS using invasive therapy in men and women. However, the risk of composite end-point was lower in biomarker (creatinine kinase-MB or troponin) positive women. Regarding potential risks associated with these invasive procedures, women have been shown to have more bleeding complications. Taken together with the less aggressive medical management, women overall have higher mortality after MI with lower health-related quality of life compared to men [116].
Women are less frequently referred for appropriate diagnostic procedures and thus may receive less therapy. Moreover, women are less often referred for cardiac rehabilitation after ACS, despite the clear benefits on overall well-being and reduction of future cardiac events [130, 131].
Therapies of Specific Conditions in Women
Treatment of microvascular angina in women starts with risk factor modification and therapeutic lifestyle changes. Exercise training and cardiac rehabilitation is often recommended. Statins, by their anti-inflammatory properties, are especially beneficial in improving endothelial function. Traditional anti-ischemic drugs, including nitrates, beta blockers, ACEI and calcium channels blockers are first line therapy. L-arginine, a precursor of nitric oxide, improves angina and improves small vessel endothelial function in nonobstructive CAD [132], although its long-term use in certain situations is being questioned. The non-traditional anti-ischemic medications including ranolazine (an anti-anginal agent) or xanthine derivatives such as aminophylline have also shown to benefit. Xanthines and tricyclics are effective also on abnormal cardiac pain perception [133]. Isolated reports of the use of cGMP phosphodiesterase inhibitors have emerged, but no consistent studies have been done.
Strategies for long-term management of coronary microvascular dysfunction in women are challenging and not well established. This is partially due to our still incomplete understanding of the pathophysiology of microvascular dysfunction and limited effectiveness of current conventional therapies. Large, randomized outcome clinical trials testing the efficacy of currently available medical therapies or novel therapies in women with refractory symptoms are lacking. Further research is needed to evaluate the best long-term treatment strategy and to provide treatment guidelines.
The role of menopausal hormone therapy (MHT) in primary prevention of CAD in women has not been confirmed and data is insufficient to recommend its use [134, 135] for the prevention (primary or secondary) of CAD. However, a recent study, Kronos Early Estrogen Study (KEEPS), exploring the use of MHT in recently menopausal women (mean age of 50, in contrast to the mean age of 63 in the Women’s Health Initiative (WHI) trial) found that there was no acceleration of atherosclerosis as detected by carotid intima media thickness and CAC score [136]. This suggests that MHT is not harmful to the cardiovascular system when clinically-indicated for treatment of vasomotor menopausal symptoms. Indeed, in perimenopausal and early menopausal women with refractory chest pain symptoms due to MVD, observational experience suggests that a trial of MHT may be beneficial in symptom relief. One could postulate that the fluctuation and withdrawal of estrogen levels at time of perimenopause could provoke untoward vasomotor effects upon the endothelium in the coronary microvasculature. However, there is currently no clear evidence base for this suggestion. Interestingly, the Danish Osteoporosis Prevention Study (DOPS) [137] provided indirect evidence for a beneficial effect of MHT on CAD risk reduction when started early in menopause. A subset analysis of the WHI data showed similarly that the youngest tertile of patients actually had a significant reduction in cardiac events and in CAC scoring [138].
There is no role for MHT in secondary prevention. The Heart and Estrogen/Progestin Replacement Study (HERS) showed no evidence of cardiovascular benefit in women with established obstructive CAD. The rate of coronary events increased in the first two years with the use of hormone replacement therapy, while in subsequent two years, the risk decreased, with no net benefit [139, 140].
Conclusions
Although we have made great strides in the reduction of CVD mortality in women through advanced medical care, state-of-the-art medical technologies and health awareness campaigns, we still have more tread to cover. The prevention, diagnosis and treatment of women with IHD remain a great challenge which ultimately leads to healthcare inequities. A complex interplay of variables contribute to this conundrum including unique risk factors and pathophysiology for IHD among women, particularly the amassed number of women with nonobstructive CAD and dysfunction of the coronary microvasculature and endothelium [2]. Our review provides a synthesis of key evidence highlighting gender disparities in the epidemiology, presentation, risk assessment, mortality and clinical diagnosis and management of women with IHD. Women have an increase in incidence of CVD events with age, although there is an emergence of events in younger women. Furthermore, women have a high CVD risk factor burden, particularly those of African-descent and are more prone to present with atypical symptomatology which contributes to underdiagnosis and increased mortality rates. Our current diagnostic strategies are inherently tailored towards identification of “classical” obstructive CAD, with subsequent catheter-based or surgical interventions. Although some women do fit into this “accepted” algorithm, they are not consistently receiving guideline-based therapy. Moreover, we do not yet have a clear understanding of what to do with the patients, the majority of whom are women, who do not fit neatly into this standard algorithm, yet have persistent symptoms, and increased morbidity and mortality.
These persistent disparities provide a framework for clinicians and researchers to “refashion” and remodel current practices in the evaluation of women with IHD with the overarching goal of providing efficient and cost-effective healthcare for improved clinical outcomes. The fundamental hurdle remains to build credible sex-specific evidence on CVD mechanisms through better representation of women in cardiovascular clinical trials. In this era of health care reform, future guidelines for the assessment of IHD in women must include gender-specific risk assessment models as well as diagnostic and therapeutic algorithms for obstructive and nonobstructive CAD.
Abbreviations used in the paper: ACC, American College of Cardiology; ACS, acute coronary syndromes; ACEI, ACE inhibitors; AHA, American Heart Association; ARB, angiotensin receptor blockers; ARIC, Atherosclerosis Risk in Communities; ASCVD, atherosclerotic cardiovascular risk; CABG, coronary artery bypass grafting; CAC, coronary artery calcium; CAD, coronary artery disease; CASS, Coronary Artery Surgery Study; CCTA, coronary computed tomographic angiography; CMR, cardiac magnetic resonance imaging; CVD, cardiovascular disease; CT, computed tomography; ETT, exercise treadmill test; FRS, Framingham risk score; hsCRP, high-sensitivity C-reactive protein; IHD, ischemic heart disease; MACE, major adverse cardiac events; MHT, menopausal hormone therapy; MI, myocardial infarction; MPI, myocardial perfusion imaging; MVD, microvascular disease; NCDR, National Cardiovascular Data Registry; NHANES, National Health and Nutrition Examination Survey; NHLBI, National Heart, Lung, and Blood Institute; PCI, percutaneous coronary intervention; PCOS, polycystic ovarian syndrome; PET, positron emission tomography; PRHI, peripheral reactive hyperemia index; SPECT, single-photon emission computed tomography; WHI, Women’s Health Initiative; WISE, Women’s Ischemia Syndrome Evaluation;
References
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the american heart association. Circulation. 2015;131:e29–e322.
Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, et al. Insights from the nhlbi-sponsored women’s ischemia syndrome evaluation (wise) study: Part i: Gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47:S4–S20.
Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–75.
Kim ES, Carrigan TP, Menon V. Enrollment of women in national heart, lung, and blood institute-funded cardiovascular randomized controlled trials fails to meet current federal mandates for inclusion. J Am Coll Cardiol. 2008;52:672–3.
Bucholz EM, Butala NM, Rathore SS, Dreyer RP, Lansky AJ, Krumholz HM. Sex differences in long-term mortality after myocardial infarction: a systematic review. Circulation. 2014;130:757–67.
Wenger NK. Women and coronary heart disease: a century after Herrick: understudied, underdiagnosed, and undertreated. Circulation. 2012;126:604–11.
Towfighi A, Zheng L, Ovbiagele B. Sex-specific trends in midlife coronary heart disease risk and prevalence. Arch Intern Med. 2009;169:1762–6.
Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, et al. Postmenopausal estrogen therapy and cardiovascular disease: ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325:756–62.
Campisi R. Noninvasive assessment of coronary microvascular function in women at risk for ischaemic heart disease. Int J Clin Pract. 2008;62:300–7.
Mack M, Gopal A. Epidemiology, traditional and novel risk factors in coronary artery disease. Cardiol Clin. 2014;32:323–32.
Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the american heart association. Circulation. 2010;121:948.
Feinstein M, Ning H, Kang J, Bertoni A, Carnethon M, Lloyd-Jones DM. Racial differences in risks for first cardiovascular events and noncardiovascular death: the atherosclerosis risk in communities study, the cardiovascular health study, and the multi-ethnic study of atherosclerosis. Circulation. 2012;126:50–9.
Ford ES, Capewell S. Coronary heart disease mortality among young adults in the us from 1980 through 2002: Concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50:2128–32.
Dreyer RP, Smolderen KG, Strait KM, Beltrame JF, Lichtman JH, Lorenze NP, et al. Gender differences in pre-event health status of young patients with acute myocardial infarction: a virgo study analysis. Eur Heart J Acute Cardiovasc Care. in press.
Zuchi C, Tritto I, Ambrosio G. Angina pectoris in women: focus on microvascular disease. Int J Cardiol. 2013;163:132–40.
Hemingway H, Langenberg C, Damant J, Frost C, Pyörälä K, Barrett-Connor E. Prevalence of angina in women versus men a systematic review and meta-analysis of international variations across 31 countries. Circulation. 2008;117:1526–36.
Mieres JH, Heller GV, Hendel RC, Gulati M, Boden WE, Katten D, et al. Signs and symptoms of suspected myocardial ischemia in women: results from the what is the optimal method for ischemia evaluation in women? Trial J Womens Health. 2011;20:1261–8.
Eastwood J-A, Johnson BD, Rutledge T, Bittner V, Whittaker KS, Krantz DS, et al. Anginal symptoms, coronary artery disease, and adverse outcomes in black and white women: the nhlbi-sponsored women’s ischemia syndrome evaluation (wise) study. J Womens Health. 2013;22:724–32.
Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the women’s ischemia syndrome evaluation study and the st james women take heart project. Arch Intern Med. 2009;169:843–50.
Tamis-Holland JE, Lu J, Korytkowski M, Magee M, Rogers WJ, Lopes N, et al. Sex differences in presentation and outcome among patients with type 2 diabetes and coronary artery disease treated with contemporary medical therapy with or without prompt revascularization: a report from the bari 2d trial (bypass angioplasty revascularization investigation 2 diabetes). J Am Coll Cardiol. 2013;61:1767–76.
Vaccarino V. Ischemic heart disease in women many questions, few facts. Circ Cardiovasc Qual Outcomes. 2010;3:111–5.
Sharaf BL, Pepine CJ, Kerensky RA, Reis SE, Reichek N, Rogers WJ, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the nhlbi-sponsored women’s ischemia syndrome evaluation [wise] study angiographic core laboratory). Am J Cardiol. 2001;87:937–41.
Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the national institutes of health--national heart, lung, and blood institute--sponsored women’s ischemia syndrome evaluation. Circulation. 2006;114:894–904.
Shaw LJ, Shaw RE, Merz CNB, Brindis RG, Klein LW, Nallamothu B, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the american college of cardiology–national cardiovascular data registry. Circulation. 2008;117:1787–801.
Maynard C, Fisher L, Passamani E, Pullum T. Blacks in the coronary artery surgery study: risk factors and coronary artery disease. Circulation. 1986;74:64–71.
Gulati M, Shaw LJ, Merz B, Noel C. Myocardial ischemia in women: lessons from the nhlbi wise study. Clin Cardiol. 2012;35:141–8.
Bransford TL, Ofili E. The paradox of coronary heart disease in african-american women. J Natl Med Assoc. 2000;92:327.
Mensah GA, Brown DW. An overview of cardiovascular disease burden in the united states. Health Aff. 2007;26:38–48.
Mosca L, Hammond G, Mochari-Greenberger H, Towfighi A, Albert MA. Fifteen-year trends in awareness of heart disease in women results of a 2012 american heart association national survey. Circulation. 2013;127:1254–63.
Papakonstantinou NA, Stamou MI, Baikoussis NG, Goudevenos J, Apostolakis E. Sex differentiation with regard to coronary artery disease. J Cardiol. 2013;62:4–11.
Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, et al. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337–45.
van Loo HM, van den Heuvel ER, Schoevers RA, Anselmino M, Carney RM, Denollet J, et al. Sex dependent risk factors for mortality after myocardial infarction: Individual patient data meta-analysis. BMC Med. 2014;12:242.
Vaccarino V, Horwitz RI, Meehan TP, Petrillo MK, Radford MJ, Krumholz HM. Sex differences in mortality after myocardial infarction: evidence for a sex-age interaction. Arch Intern Med. 1998;158:2054–62.
Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. N Engl J Med. 1999;341:217–25.
Berger JS, Brown DL. Gender-age interaction in early mortality following primary angioplasty for acute myocardial infarction. Am J Cardiol. 2006;98:1140–3.
Johnson BD, Shaw LJ, Pepine CJ, Reis SE, Kelsey SF, Sopko G, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the nih-nhlbi-sponsored women’s ischaemia syndrome evaluation (wise) study. Eur Heart J. 2006;27:1408–15.
Banks K, Lo M, Khera A. Angina in women without obstructive coronary artery disease. Curr Cardiol Rev. 2010;6:71.
Handberg EM, Eastwood JA, Eteiba W, Johnson BD, Krantz DS, Thompson DV, et al. Clinical implications of the women’s ischemia syndrome evaluation: Inter-relationships between symptoms, psychosocial factors and cardiovascular outcomes. Womens Health (Lond Engl). 2013;9:479–90.
Norris CM, Spertus JA, Jensen L, Johnson J, Hegadoren KM, Ghali WA. Sex and gender discrepancies in health-related quality of life outcomes among patients with established coronary artery disease. Circ Cardiovasc Qual Outcomes. 2008;1:123–30.
Vaccarino V, Lin ZQ, Kasl SV, Mattera JA, Roumanis SA, Abramson JL, et al. Gender differences in recovery after coronary artery bypass surgery. J Am Coll Cardiol. 2003;41:307–14.
Scheuner MT, Setodji CM, Pankow JS, Blumenthal RS, Keeler E. Relation of familial patterns of coronary heart disease, stroke, and diabetes to subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. Genet Med. 2008;10:879–87.
Kannel WB. Metabolic risk factors for coronary heart disease in women: perspective from the Framingham study. Am Heart J. 1987;114:413–9.
Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9.
Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–73.
Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383–90.
Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a metaanalysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–9.
Reuterwall C, Hallqvist J, Ahlbom A, De Faire U, Diderichsen F, Hogstedt C, et al. Higher relative, but lower absolute risks of myocardial infarction in women than in men: analysis of some major risk factors in the sheep study. The sheep study group. J Intern Med. 1999;246:161–74.
Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378:1297–305.
Steliga MA, Dresler CM. Smoking cessation: crucial to target women as well as men. Lancet. 2011;378:1278–9.
Sharma K, Gulati M. Coronary artery disease in women: a 2013 update. Glob Health. 2013;8:105–12.
Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–9.
Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, et al. Trends in cardiovascular health metrics and associations with all-cause and cvd mortality among us adults. JAMA. 2012;307:1273–83.
Kling JM, Miller VM, Mankad R, Wilansky S, Wu Q, Zais TG, et al. Go red for women cardiovascular health-screening evaluation: the dichotomy between awareness and perception of cardiovascular risk in the community. J Womens Health (Larchmt). 2013;22:210–8.
Kip KE, Marroquin OC, Kelley DE, Johnson BD, Kelsey SF, Shaw LJ, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the women’s ischemia syndrome evaluation (wise) study. Circulation. 2004;109:706–13.
Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-hdl cholesterol, apolipoproteins a-i and b100, standard lipid measures, lipid ratios, and crp as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–33.
Wong ND, Pio J, Valencia R, Thakal G. Distribution of c-reactive protein and its relation to risk factors and coronary heart disease risk estimation in the national health and nutrition examination survey (nhanes) iii. Prev Cardiol. 2001;4:109–14.
Karim R, Stanczyk FZ, Hodis HN, Cushman M, Lobo RA, Hwang J, et al. Associations between markers of inflammation and physiological and pharmacological levels of circulating sex hormones in postmenopausal women. Menopause. 2010;17:785–90.
Salmon JE, Roman MJ. Subclinical atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Am J Med. 2008;121:010.
Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94:4953–60.
Bairey Merz CN, Johnson BD, Sharaf BL, Bittner V, Berga SL, Braunstein GD, et al. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the nhlbi-sponsored wise study. J Am Coll Cardiol. 2003;41:413–9.
Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation. 2011;57:1404–23.
Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (champs): population-based retrospective cohort study. Lancet. 2005;366:1797–803.
Han SH, Bae JH, Holmes Jr DR, Lennon RJ, Eeckhout E, Barsness GW, et al. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J. 2008;29:1359–69.
Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the nhlbi wise study. Am Heart J. 2001;141:735–41.
Kothawade K, Bairey Merz CN. Microvascular coronary dysfunction in women: pathophysiology, diagnosis, and management. Curr Probl Cardiol. 2011;36:291–318.
Bairey Merz CN, Pepine CJ. Syndrome x and microvascular coronary dysfunction. Circulation. 2011;124:1477–80.
von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the national heart, lung, and blood institute-sponsored women’s ischemia syndrome evaluation (wise). Circulation. 2004;109:722–5.
Houghton JL, Smith VE, Strogatz DS, Henches NL, Breisblatt WM, Carr AA. Effect of african-american race and hypertensive left ventricular hypertrophy on coronary vascular reactivity and endothelial function. Hypertension. 1997;29:706–14.
Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes Jr DR, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54.
Madhok V, Fahey T. Cardiovascular risk estimation: important but may be inaccurate. BMJ. 2006;332(7555):1422.
Kones R. Primary prevention of coronary heart disease: integration of new data, evolving views, revised goals, and role of rosuvastatin in management. A comprehensive survey. Drug Des Devel Ther. 2011;5:325–80.
Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the score project. Eur Heart J. 2003;24:987–1003.
Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ. 2007;335(7611):136.
NCEP. Third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation. 2002;106:3143.
Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds risk score. JAMA. 2007;297:611–9.
Wenger NK. The Reynolds risk score: improved accuracy for cardiovascular risk prediction in women? Nat Clin Pract Cardiovasc Med. 2007;4:366–7.
Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 acc/aha guideline on the assessment of cardiovascular risk: a report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129:S49–73.
Gulati M, Noel Bairey Merz C. New cholesterol guidelines and primary prevention in women. Trends Cardiovasc Med. 2014;25:84–94.
Ridker PM, Cook NR. Statins: New american guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762–5.
Cook NR, Ridker PM. Further insight into the cardiovascular risk calculator: the roles of statins, revascularizations, and underascertainment in the women’s health study. JAMA Intern Med. 2014;174:1964–71.
Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307:813–22.
Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GBJ, Feit F, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–25.
Abdelmoneim SS, Bernier M, Dhoble A, Moir S, Hagen ME, Ness SAC, et al. Assessment of myocardial perfusion during adenosine stress using real time three-dimensional and two-dimensional myocardial contrast echocardiography: comparison with single-photon emission computed tomography. Echocardiography. 2010;27:421–9.
Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579.
Yip A, Saw J. Spontaneous coronary artery dissection-a review. Cardiovasc Diagn Ther. 2015;5:37–48.
Mieres JH, Gulati M, Bairey Merz N, Berman DS, Gerber TC, Hayes SN, et al. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the american heart association. Circulation. 2014;130:350–79.
Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, et al. Exercise capacity and the risk of death in women: the st james women take heart project. Circulation. 2003;108:1554–9.
Bourque JM, Holland BH, Watson DD, Beller GA. Achieving an exercise workload of≥ 10 metabolic equivalents predicts a very low risk of inducible ischemia: does myocardial perfusion imaging have a role? J Am Coll Cardiol. 2009;54:538–45.
Kohli P, Gulati M. Exercise stress testing in women: going back to the basics. Circulation. 2010;122:2570–80.
Hlatky MA, Pryor DB, Harrell Jr FE, Califf RM, Mark DB, Rosati RA. Factors affecting sensitivity and specificity of exercise electrocardiography. Multivariable analysis. Am J Med. 1984;77:64–71.
Alexander KP, Shaw LJ, Shaw LK, Delong ER, Mark DB, Peterson ED. Value of exercise treadmill testing in women. J Am Coll Cardiol. 1998;32:1657–64.
Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical-diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–8.
Marwick TH, Nemec JJ, Pashkow FJ, Stewart WJ, Salcedo EE. Accuracy and limitations of exercise echocardiography in a routine clinical setting. J Am Coll Cardiol. 1992;19:74–81.
Williams MJ, Marwick TH, O’Gorman D, Foale RA. Comparison of exercise echocardiography with an exercise score to diagnose coronary artery disease in women. Am J Cardiol. 1994;74:435–8.
Kim C, Kwok YS, Heagerty P, Redberg R. Pharmacologic stress testing for coronary disease diagnosis: a meta-analysis. Am Heart J. 2001;142:934–44.
Roger VL, Pellikka PA, Bell MR, Chow CW, Bailey KR, Seward JB. Sex and test verification bias. Impact on the diagnostic value of exercise echocardiography. Circulation. 1997;95:405–10.
Arruda-Olson AM, Juracan EM, Mahoney DW, McCully RB, Roger VL, Pellikka PA. Prognostic value of exercise echocardiography in 5,798 patients: Is there a gender difference? J Am Coll Cardiol. 2002;39:625–31.
McCully RB, Roger VL, Mahoney DW, Karon BL, Oh JK, Miller Jr FA, et al. Outcome after normal exercise echocardiography and predictors of subsequent cardiac events: follow-up of 1,325 patients. J Am Coll Cardiol. 1998;31:144–9.
Gerber TC, Gibbons RJ. Weighing the risks and benefits of cardiac imaging with ionizing radiation. JACC Cardiovasc Imaging. 2010;3:528–35.
Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the international multicenter confirm (coronary ct angiography evaluation for clinical outcomes: an international multicenter registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–60.
Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: The romicat (rule out myocardial infarction using computer assisted tomography) trial. J Am Coll Cardiol. 2009;53:1642–50.
Schlett CL, Banerji D, Siegel E, Bamberg F, Lehman SJ, Ferencik M, et al. Prognostic value of ct angiography for major adverse cardiac events in patients with acute chest pain from the emergency department: 2-year outcomes of the romicat trial. JACC Cardiovasc Imaging. 2011;4:481–91.
Devries S, Wolfkiel C, Fusman B, Bakdash H, Ahmed A, Levy P, et al. Influence of age and gender on the presence of coronary calcium detected by ultrafast computed tomography. J Am Coll Cardiol. 1995;25:76–82.
Budoff MJ, Shokooh S, Shavelle RM, Kim HT, French WJ. Electron beam tomography and angiography: sex differences. Am Heart J. 2002;143:877–82.
Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291–300.
Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J. 2012;33:2771–2782b.
Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease. JACC Cardiovasc Interv. 2012;5:646–53.
Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the national heart, lung and blood institute wise (women’s ischemia syndrome evaluation) study. J Am Coll Cardiol. 2010;55:2825–32.
Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51:997–1002.
Bonetti PO, Pumper GM, Higano ST, Holmes Jr DR, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–41.
Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, et al. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55:1688–96.
Sedlak TL, Lee M, Izadnegahdar M, Merz CNB, Gao M, Humphries KH. Sex differences in clinical outcomes in patients with stable angina and no obstructive coronary artery disease. Am Heart J. 2013;166:38–44.
Lerman A, Sopko G. Women and cardiovascular heart disease: clinical implications from the women’s ischemia syndrome evaluation (wise) study. Are we smarter? J Am Coll Cardiol. 2006;47:S59–62.
Hasdai D, Cannan CR, Mathew V, Holmes Jr DR, Lerman A. Evaluation of patients with minimally obstructive coronary artery disease and angina. Int J Cardiol. 1996;53:203–8.
Amsterdam EA, Wenger NK, Brindis RG, Casey Jr DE, Ganiats TG, Holmes Jr DR, et al. 2014 aha/acc guideline for the management of patients with non-st-elevation acute coronary syndromes: a report of the american college of cardiology/american heart association task force on practice guidelines. J Am Coll Cardiol. 2014;64:e139–228.
Blomkalns AL, Chen AY, Hochman JS, Peterson ED, Trynosky K, Diercks DB, et al. Gender disparities in the diagnosis and treatment of non-st-segment elevation acute coronary syndromes: large-scale observations from the crusade (can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the american college of cardiology/american heart association guidelines) national quality improvement initiative. J Am Coll Cardiol. 2005;45:832–7.
Gehrie ER, Reynolds HR, Chen AY, Neelon BH, Roe MT, Gibler WB, et al. Characterization and outcomes of women and men with non-st-segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the acc/aha guidelines (crusade) quality improvement initiative. Am Heart J. 2009;158:688–94.
Daly C, Clemens F, Lopez Sendon JL, Tavazzi L, Boersma E, Danchin N, et al. Gender differences in the management and clinical outcome of stable angina. Circulation. 2006;113:490–8.
Dey S, Flather MD, Devlin G, Brieger D, Gurfinkel EP, Steg PG, et al. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the global registry of acute coronary events. Heart. 2009;95:20–6.
Steg PG, Greenlaw N, Tardif JC, Tendera M, Ford I, Kaab S, et al. Women and men with stable coronary artery disease have similar clinical outcomes: insights from the international prospective clarify registry. Eur Heart J. 2012;33:2831–40.
Collaborative overview of randomised trials of antiplatelet therapy--i: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet trialists’ collaboration. BMJ. 1994;308:81–106.
Chan AT, Manson JE, Feskanich D, Stampfer MJ, Colditz GA, Fuchs CS. Long-term aspirin use and mortality in women. Arch Intern Med. 2007;167:562–72.
Becker DM, Segal J, Vaidya D, Yanek LR, Herrera-Galeano JE, Bray PF, et al. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA. 2006;295:1420–7.
Ridker PM, Hennekens CH, Tofler GH, Lipinska I, Buring JE. Anti-platelet effects of 100 mg alternate day oral aspirin: a randomized, double-blind, placebo-controlled trial of regular and enteric coated formulations in men and women. J Cardiovasc Risk. 1996;3:209–12.
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto Jr AM, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. N Engl J Med. 2008;359:2195–207.
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22.
Lewis SJ, Sacks FM, Mitchell JS, East C, Glasser S, Kell S, et al. Effect of pravastatin on cardiovascular events in women after myocardial infarction: the cholesterol and recurrent events (care) trial. J Am Coll Cardiol. 1998;32:140–6.
Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504.
O’Donoghue M, Boden WE, Braunwald E, Cannon CP, Clayton TC, de Winter RJ, et al. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-st-segment elevation myocardial infarction: a meta-analysis. JAMA. 2008;300:71–80.
Lavie CJ, Milani RV. Effects of cardiac rehabilitation and exercise training on exercise capacity, coronary risk factors, behavioral characteristics, and quality of life in women. Am J Cardiol. 1995;75:340–3.
Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond a presidential advisory from the american heart association. Circulation. 2011;124:2951–60.
Lerman A, Burnett Jr JC, Higano ST, McKinley LJ, Holmes Jr DR. Long-term l-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123–8.
Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–11.
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA. 2002;288:321–33.
Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the women’s health initiative randomized controlled trial. JAMA. 2004;291:1701–12.
Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014;161:249–60.
Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. 2012;345:e6409.
Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, et al. Conjugated equine estrogens and coronary heart disease - the women’s health initiative. Arch Intern Med. 2006;166:357–65.
Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and estrogen/progestin replacement study (hers) research group. JAMA. 1998;280:605–13.
Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: heart and estrogen/progestin replacement study follow-up (HERS II). JAMA. 2002;288:49–57.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brewer, L.C., Svatikova, A. & Mulvagh, S.L. The Challenges of Prevention, Diagnosis and Treatment of Ischemic Heart Disease in Women. Cardiovasc Drugs Ther 29, 355–368 (2015). https://doi.org/10.1007/s10557-015-6607-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-015-6607-4