It is shown that the common practice of evaluating corrosion resistance based on specimen weight loss does not provide a reliable indication of the protective properties of metallic coatings; use of the results obtained by this method does not guarantee the absence of local damage to the base metal. A simple and reliable new method is proposed for evaluating such coatings’ protective properties. The method entails performing a qualitative analysis of the medium in which coated specimens are corrosion-tested in order to detect any iron ions that are present. The new method provides information on coating quality that adds to the information which can be obtained by standard methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Metallic coatings have been used more and more in recent years to protect the equipment inside wells. The protective properties of the coatings are evaluated by the methods stipulated in the standards GOST 9.304–87, Thermal-Spray Coatings, GOST 9.308–85, Metallic and Nonmetallic Inorganic Coatings. Methods for Accelerated Corrosion-Testing, GOST 9.311–87, Metallic and Nonmetallic Inorganic Coatings. Methods of Evaluating Corrosion Damage, and other regulations. These standards provide for a wide range of tests under different conditions over long periods – up to 2–3 years – and short periods – several hours. The protective properties of the coatings are judged based on changes in their appearance. If those changes are small, then the evaluation is made based on the loss of weight by the specimen, its change in electrical conductivity, the relative area damaged by corrosion, or other criteria. The relative area of the corrosion damage is used to evaluate the protective properties even of cathodic coatings. In our opinion, methods such as those just mentioned are acceptable for decorative coatings. However, they are not adequate for determining the protective properties of coatings on equipment that is used underground. The coating acts as a protector when it is anodic, i.e., when the coating’s electrode potential is more negative than the potential of the metal being protected. It is known that corrosion rate is proportional to the density of the corrosion current rather than its magnitude. Thus, if an anodic coating contains a small through pore, the exposed section of the metal being protected will act as a cathode and the surface of the coating will act as an anode. In this case, the area of the anode (the dissolving electrode in the corrosion cell) will be incomparably greater than the area of the cathodic section. Thus, the coating dissolves at a very low rate and the exposed section that is acting as the cathode does not dissolve at all. The opposite situation prevails in the case of a cathodic coating. If even a very small section of the metal that is being protected becomes exposed, that section is converted into an anode and undergoes active dissolution because it is incomparably small compared to the cathode (the coating). Localized damage takes place and subsequently serves as a stress concentrator. In a metal structural element under tensile loads, the concentrator will initiate a corrosion crack. This dangerous defect may not be visually observable and can go undetected.
The goal of our investigation was to develop a simple and easy-to-use method of detecting through-type defects in protective metallic coatings.
The objects of investigation were carbon-steel (steel St 3) specimens with hard-facings composed of the following materials: steels SS410L (03Kh13) and SS316L (Kh17N13M3T) and alloys 1560 (nickel-based), 2537-10 (cobalt-based), and CuSn11 (bronze with tin as its main additive). Table 1 shows the chemical composition of the hard-facing materials.
The working media were prepared from chemically pure reagents NaCl, NaOH, and CH2COOH. The test medium was a 3% solution of NaCl acidified to pH = 3.
0.5-N solutions of K3[Fe(CN)6] and K4[Fe(CN)6] were used as indicators to detect the presence of iron ions.
The tests were performed using the following equipment: electronic analytical balance Vibra HT HTR-120CE; millivoltmeter Ekspert-pH, which was used as the pH-meter; cutter MICROMET M3/200-SAS; drying cabinet LABOR MUSZERIPARI MUVEK LP-301; grinder-polisher APOL L62/LSA6-40-SAS; silver-chloride reference electrodes EVL-1M3.1; inverted metallographic microscope NIKON MA20.
Experimental Method and Discussion of the Results. First, we determined the rate of corrosion of metal specimens with and without a hard-facing. Corrosion rate was determined in the above-indicated solutions in accordance with the standard GOST 9.908, Metals and Alloys. Methods of Determining Corrosion and Corrosion-Resistance Indices. In keeping with the requirements of the customer, the test base was limited to 96 h. The corrosion process was accelerated by the injection of air to form bubbles in the test media. Again following the customer’s requirements, the protective properties of the coatings were determined based on corrosion rate by comparing uncoated and coated specimens.
Table 2 shows the results obtained from tests of the metallic hard-facings.
The data shows that, to a greater or lesser degree, all the coatings except steel 03Kh13 protected the base metal.
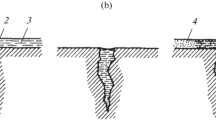
Visual inspection of the coated specimens after the tests (Fig. 1) revealed the presence of corrosion products in the form of iron oxides (friable ginger-colored oxides) on the specimens coated with steels 03Kh13 and Kh17N13M3T and the nickel alloy. The corrosion products on the specimens with bronze coatings resembled oxides of copper – dense and black. No corrosion products were seen on the specimens with the cobalt coating.
Metallographic analysis of the specimens after the tests (Fig. 2) showed the presence of corrosion products in the coating of steel 03Kh13. No corrosion damage was detected on the specimens with coatings of steel Kh17N13M3T or the cobalt alloy. Corrosion products were found on the outside surface of the bronze-coated specimens, while the specimens with the nickel-based coating contained through pores that led to highly localized damage to the base metal.
At first glance, the cobalt-based coating and the coating made of steel Kh17N13M3T seem to have satisfactory protective properties. First of all, however, cobalt – just as nickel and copper – acts as a cathode to iron in electrolyte solutions in the presence of electrochemical contact. Similarly, steels of type Kh17N13M3T act as a cathode to carbon steels. Secondly, the above-indicated test base was too small to allow damage on highly corrosion-resistant coatings to be detected by visual means.
Values for the electrode potentials of the base metal and coating metal in the test solutions were determined for the case of a silver-chloride reference electrode (Table 3).
It is apparent that all of the coatings except for steel 03Kh13 – which did not exhibit any protective properties – are cathodic in nature.
We collected samples of the electrolyte during the corrosion tests and acidified them to pH = 2. We then used 0.5-N solutions of K3(Fe(CN)6] and K4[Fe(CN)6] to analytically determine the presence of iron ions in the samples. Twelve hours after the beginning of the tests, iron ions were detected in the cells that included the specimens with hard-facings made of the steels and the nickel and cobalt alloys. Visual observations made during the metallographic studies did not reveal any corrosion damage to the specimens with the steel Kh17N13M3T coating or the cobalt coating. Thus, the locally damaged section was not included when we prepared the metallographic sections. No iron ions were detected in the media used for tests of the bronze-coated specimens even after 300 h of testing, which shows that those specimens did not contain any through pores. The fact that iron ions were found in the media used to test the specimens coated with the nickel and cobalt alloys shows that some of the base metal had dissolved. The presence of iron ions on the specimens coated with steel Kh17N13M3T shows that the passive state of the metal of this coating was disturbed, protective properties were lost, and the coating contained through pores. In any case, specimens for which iron ions are found in the test medium cannot be recommended for use.
The results obtained from our investigations were used to propose a simple and reliable method of evaluating the integrity of metallic coatings. The method entails analyzing the medium in which coated specimens are corrosion-tested in order to detect any iron ions that are present. Our studies showed that just 12 h of testing is sufficient to detect iron ions if the metal of the coating contains through pores. Analyzing the test medium can be regarded as an additional means of evaluating the protective properties of coatings. This method can be used in combination with standard test methods and metallographic evaluation of the characteristics of coatings.
Author information
Authors and Affiliations
Additional information
Translated from Khimicheskoe i Neftegazovoe Mashinostroenie, No. 9, pp. 41–43, September, 2012.
Rights and permissions
About this article
Cite this article
Medvedeva, M.L., Prygaev, A.K., Baklanova, Y.V. et al. Method of finding through-type defects in metallic coatings. Chem Petrol Eng 48, 589–592 (2013). https://doi.org/10.1007/s10556-013-9662-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10556-013-9662-3