Abstract
The expression “lost at sea” means to be confused or perplexed. By extension, lost at SCLC references the current confusion about how to circumvent the chemoresistance, particularly platinum resistance, which so plagues the treatment of extensive-stage small cell lung cancer (ES-SCLC) that in 2012 the US National Cancer Institute (NCI) designated it a “recalcitrant cancer.” Over a decade later, despite the approval of immune checkpoint inhibitors and the conditional approval of lurbinectedin, the prognosis for ES-SCLC, and especially platinum-resistant ES-SCLC, has scarcely improved. The focus of this review, which briefly summarizes current treatment options for ES-SCLC, is on five clinical-stage therapies with the potential to successfully reverse the platinum resistance that is perhaps the biggest obstacle to better clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Small cell lung cancer (SCLC), which accounts for about 15% of all lung cancer diagnoses, is an aggressive neuroendocrine cancer with limited treatment options and a dismally low overall survival rate of less than 5% at 36 months. Most, but not all, of the 150,000 patients diagnosed worldwide each year respond well to front-line platinum-based doublet chemotherapy but invariably relapse with drug-resistant disease and poor prospects for survival [1].
SCLC is virtually synonymous with tobacco abuse since 98% of cases arise in current or former heavy smokers (≥ 40 pack years). In the USA, as evidence of the direct link between SCLC and the carcinogenicity of tobacco, the incidence of the disease has decreased in parallel with cigarette use and with the widespread emergence of filtered cigarettes [2]. Other risk factors for SCLC worldwide and the main ones for never smokers are long-term exposure to indoor radon, second-hand smoke, and air pollution like nitrogen dioxide and particulate matter [3]. The exceptionally fast-doubling time of SCLC, combined with the propensity for early, widespread metastases that are not amenable to surgical resection [2], the ubiquity of comorbidities associated with tobacco smoking such as chronic obstructive pulmonary disease (COPD) and cardiovascular disease, and the insurmountability of rapidly evolved chemoresistance make it the most lethal lung cancer subtype and one of the most notoriously hard to manage. The brain, liver, adrenal glands, bones, or bone marrow is the most common sites of metastasis [4].
In practice, although the National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) guidelines recommend the incorporation of tumor-node-metastasis (TNM) staging criteria, SCLC is most typically classified as limited or extensive. Around 30% of patients present with limited stage (LS) confined to the ipsilateral hemithorax and a single radiation field [5]. The majority (70%) are diagnosed with extensive-stage disease (ES), which extends beyond the confines of the ipsilateral hemithorax and a single radiation field [6]. The median overall survival (OS) for LS-SCLC is better than for ES-SCLC, around 17 months versus 8–13 months, respectively. Despite initial response rates of up to 80% in first line, the median survival for ES-SCLC patients with disease recurrence is 4–5 months [1, 4, 6].
3 Treatment for first-line ES-SCLC
The standard first-line treatment of ES-SCLC is four to six cycles of platinum and etoposide chemotherapy, with a general preference for carboplatin over cisplatin because of less toxicity, plus the programmed death-ligand 1 (PD-L1) blocking antibodies, atezolizumab, or durvalumab based on the Impower 133 and CASPIAN clinical trials, respectively [9]. For patients at risk of chemotherapy-induced neutropenia, prophylactic treatment with granulocyte colony-stimulating factor (G-CSF) or trilaciclib, a recently approved cyclin-dependent kinase 4/6 inhibitor, is potentially indicated [10].
4 Treatment-free interval (TFI) and second-line treatment
The choice of second line and subsequent regimens depends on performance status and the length of the treatment free interval (TFI). The TFI is a measure of the time between the last platinum dose and relapse or disease progression, which strongly predicts outcome. For patients with platinum sensitive disease, which ESMO guidelines define as a TFI ≥ 90 days, options include rechallenge with a platinum-based regimen, if performance status allows, or single-agent topotecan or lurbinectedin. For patients with platinum resistant disease (TFI ≤ 90 days), the alkylator, lurbinectedin, is the only FDA-indicated agent for disease that has no response to first-line chemotherapy [11].
Note that according to NCCN Guidelines, the TFI cutoff is 6 months (180 days) for sensitive SCLC and less than 6 months (180 days) for resistant SCLC. In any case, the expected response rates for platinum-resistant disease (≤ 10%) are lower than for platinum-sensitive disease (approximately 25%) [4]. Patients who do not respond or who progress during platinum chemotherapy are designated as platinum-refractory, and this designation correlates with the poorest outcomes. Topotecan, a topoisomerase I inhibitor, is only approved for chemotherapy-sensitive, relapsed SCLC. Irinotecan, another topoisomerase I inhibitor, is sometimes used in place of topotecan since dosing is less frequent and rates of myelosuppression are lower [11].
5 Second-line and beyond treatment
The treatment strategy beyond second line is unknown with no third-line (3L) regimen regulatory approvals in Europe and the USA although the orally administered tyrosine kinase inhibitor (TKI), anlotinib, is approved in China [11]. The FDA-revoked approval for the PD-1 inhibitor, nivolumab, in 3L SCLC after phase 3 randomized trial data failed to confirm an OS benefit [12]. However, in the absence of any other approved therapies, the NCCN SCLC Panel still recommends subsequent therapy with a PD-1 inhibitor for platinum-sensitive patients that did not progress on first-line atezolizumab or durvalumab [4]. Given the lack of an a priori best option, many patients may opt to enroll on a clinical trial.
A treatment algorithm for recurrent ES-SCLC is shown in Fig. 1.
This leaves open the question of how—and with what exactly—to treat platinum-resistant patients since currently only the alkylating agent, lurbinectedin, based on single-arm clinical trial data is FDA-indicated for it. However, lurbinectedin failed to improve overall survival in a phase 3 clinical trial with doxorubicin vs. CAV (cyclophosphamide/doxorubicin/vincristine) or topotecan [10]. Below, we review six clinical-stage therapies/regimens with the potential to treat platinum-resistant disease more effectively based on reported results.
6 Tarlatamab (AMG 757)
This is a bispecific T-cell engager molecule, which binds both the inhibitory notch ligand deltalike ligand 3 (DLL3) on tumor cells and cluster of differentiation 3 (CD3) on T cells to stimulate T-cell tumor lysis [12].
In a recent phase 2 study (DeLLphi-301) of 220 SCLC patients who had received a median of two lines of treatment, 40% and 32% of patients achieved an objective response with 10 mg (n = 134) and 100 mg (n = 88) dosages of tarlatamab, respectively. Among responders, the median duration of response was at least 6 months in 59% of patients. Median progression-free survival was 4.9 months with the 10-mg dose and 3.9 months with the 100-mg dose. The 9-month estimated overall survival rates were close to 70% in both the 10-mg and 100-mg dose groups. Based on these results, the FDA granted priority review to tarlatamab in SCLC.
As the one black mark on the trial, 49% and 56% in the 10 mg and 100 mg groups, respectively, experienced cytokine release syndrome, which required intensive inpatient monitoring, although only < 3% of patients discontinued treatment because of it. A phase 3 trial (DeLLphi-304) is ongoing to compare tarlatamab in 2L SCLC at a dose of 10 mg every 2 weeks to the local standard of care, either lurbinectedin, topotecan, or amrubicin [12].
Neither DeLLphi-301 actively excluded (or included) patients with platinum resistance, and it is unknown whether they were among the responders; however, the likelihood is in favor because of the high ORR of around 40%. It is also unknown whether the presence of DLL3 is required for activity.
7 ABBV-706
ABBV-706 is an intravenously administered anti-seizure-related (SEZ) 6 monoclonal antibody conjugated to a topoisomerase 1 cytotoxic payload. In a phase 1, first-in-human clinical trial with ABBV-706 as monotherapy or with budigalimab, a programmed death 1 (PD-1) inhibitor, or carboplatin, or cisplatin, the objective response rate for previously platinum progressed SCLC patients that received three lines of therapy on average was 60% (14/23), likely indicative of platinum sensitization. The safety profile was manageable with two dose-limiting toxicities: 1 grade 4 leukopenia and neutropenia lasting > 7 days and 1 grade 4 thrombocytopenia [13].
8 RRx-001 (nibrozetone)
A bispecific small molecule, RRx-001 is a hypoxic nitric oxide (NO) donor with epigenetic activity and an inhibitor of the NLRP3 inflammasome [14,15,16]. In a single-arm phase 2 study, 26 SCLC patients who previously received 2 or more therapies were treated with 4 mg weekly RRx001 until progression followed by re-challenge with a platinum doublet from 1 L. 19/26 patients (73.1%) had platinum-resistant disease. The estimated median and 12-month OS were 8.6 months and 44.1%, respectively [17]. Based on these results, RRx-001 began a large phase 3 study called REPLATINUM in third-line or beyond SCLC in China and the US, which is well underway. As a potential treatment option for third-line and beyond ES SCLC, because of its unique platinum-sensitizing potential, RRx-001 may preferentially benefit platinum-resistant patients. Unlike topotecan, lurbinectedin, and FOLFIRI, RRx-001 is non-myelosuppressive with transient pain on infusion as its main, and arguably only, side effect [18, 19]. RRx-001 has received FDA Fast Track designation for the treatment of severe oral mucositis.
9 DS7300a (ifinatamab deruxtecan, I-DXd)
DS7300a is a B7-H3-specific monoclonal antibody (mAb) conjugated to DXd, a DNA topoisomerase I inhibitor. In a phase 1/2 clinical trial, 22 patients who had received a median of two prior lines of therapy with SCLC unselected for B7-H3 expression were treated with I-DXd. With 52.4% confirmed responses, and 4.8% complete responses, the median progression-free survival was 5.8 months and the median overall survival was 12.2 months. It is unknown whether platinum-resistant patients were among the responders [20].
10 Conclusions
Platinum-based chemotherapy is a mainstay of treatment for approximately half of all tumor types; these include SCLC, testicular cancer, bladder cancer, ovarian cancer, urothelial cancer, cervical and endometrial cancer, biliary cancer, esophageal cancer, mesothelioma, testicular germ cell tumors, non-small cell lung cancer (NSCLC), melanoma, and lymphomas [21,22,23]. Equally pervasive as the use of platinum-based chemotherapy is the primary and secondary resistance that initially presents or develops to it. Therefore, the onus is on the oncology community to identify and develop therapies with the potential to circumvent platinum resistance for better outcomes and quality of life.
Approximately 20% of ES-SCLC patients are platinum resistant. Options for ES-SCLC patients are limited to begin with, and platinum-resistant disease further limits those options. In poker terms, platinum-sensitive patients hold “stronger hands” than their platinum-resistant (PR) counterparts who, with few to no cards left to play, may choose to “fold ‘em” and enter hospice for comfort care or to take their chances with potential 2L therapeutic options like lurbinectedin (the only FDA-approved agent for PR), topotecan, cyclophosphamide, adriamycin, and vincristine (CAV), paclitaxel, irinotecan, and temozolomide (or a clinical trial) despite significantly inferior response rates.
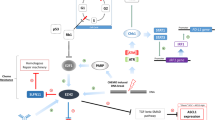
The complex and incompletely understood mechanisms of platinum resistance, the exploration of which exceeds the narrow parameters of this review, include loss of tumor suppressor genes, retinoblastoma 1 (RB1) and tumor protein p53 (TP53), poor blood flow and poor drug delivery, increased DNA repair, upregulation of the oncogene myc, multidrug resistance (MDR), epigenetic modification, and platinum inactivation, as illustrated in Figs. 2 and 3 [24].
Further elucidation of these mechanisms has the potential to improve treatment outcomes, but, as always with SCLC, to borrow a pathology phrase, “tissue is the issue” since the rarity of surgical resection, the rapidity of disease progression, and the prevalence of patient comorbidities make it difficult to obtain enough tissue biopsies [25]. In the meantime, liquid biopsies including circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) may fill the gap due to their lack of invasiveness, ease of access, and good repeatability.
Having been lost at SCLC for over 2 decades since the introduction of cisplatin/etoposide and topotecan in the 1980s and 1990s, respectively, small cell lung cancer has found itself amid a revival of sorts with a spate of several approvals in first and second line. See the timeline of approvals above.
This revival may continue with the five clinical-stage therapies/regimens discussed in this review, all of which are possibly close to approval. Even more exciting is their crossover potential to sensitize not just SCLC but also multiple other platinum-resistant tumor types and engender hope and expectancy where none (or almost none) was available before.
Data availability
No datasets were generated or analysed during the current study.
References
Oronsky, B., Reid, T. R., Oronsky, A., & Carter, C. A. (2017). What’s new in SCLC? A review. Neoplasia., 19(10), 842–847. https://doi.org/10.1016/j.neo.2017.07.007
Hou, J. M., Krebs, M. G., Lancashire, L., et al. (2012). Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. Journal of Clinical Oncology, 30(5), 525–532. https://doi.org/10.1200/JCO.2010.33.3716
Torres-Durán, M., Curiel-García, M. T., Ruano-Ravina, A., et al. (2021). Small-cell lung cancer in never-smokers. ESMO Open., 6(2), 100059. https://doi.org/10.1016/j.esmoop.2021.100059
Oronsky, B., Abrouk, N., Caroen, S., Lybeck, M., Guo, X., Wang, X., Yu, Z., & Reid, T. (2022). A 2022 update on extensive stage small-cell lung cancer (SCLC). Journal of Cancer, 13(9), 2945–2953. https://doi.org/10.7150/jca.75622
Ganti, A. K. P., Loo, B. W., Bassetti, M., et al. (2021). Small cell lung cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network, 19(12), 1441–1464. https://doi.org/10.6004/jnccn.2021.0058
Gergen, A. K., Scott, C. D., & Mitchell, J. D. (2020). Surgery for limited stage small cell lung cancer. Journal of Thoracic Disease, 12(10), 6291–6297. https://doi.org/10.21037/jtd.2020.03.79
Blackhall, F., Girard, N., Livartowski, A., et al. (2023). Treatment patterns and outcomes among patients with small-cell lung cancer (SCLC) in Europe: A retrospective cohort study. British Medical Journal Open, 13(2), e052556. https://doi.org/10.1136/bmjopen-2021-052556
Bogart, J. A., Waqar, S. N., & Mix, M. D. (2022). Radiation and systemic therapy for limited-stage small-cell lung cancer. Journal of Clinical Oncology, 40(6), 661–670. https://doi.org/10.1200/JCO.21.01639
Rittberg, R., Leung, B., Al-Hashami, Z., et al. (2022). Real-world eligibility for platinum doublet plus immune checkpoint inhibitors in extensive-stage small-cell lung cancer. Frontiers in Oncology, 15(12), 1002385. https://doi.org/10.3389/fonc.2022.1002385
Ferrarotto, R., Anderson, I., Medgyasszay, B., et al. (2021). Trilaciclib prior to chemotherapy reduces the usage of supportive care interventions for chemotherapy-induced myelosuppression in patients with small cell lung cancer: Pooled analysis of three randomized phase 2 trials. Cancer Medicine, 10(17), 5748–5756. https://doi.org/10.1002/cam4.4089
Kondo, R., Watanabe, S., Shoji, S., et al. (2018). A phase II study of irinotecan for patients with previously treated small-cell lung cancer. Oncology, 94, 223–232. https://doi.org/10.1159/000486622
Ahn, M. J., Cho, B. C., Felip, E., et al. (2023). Tarlatamab for patients with previously treated small cell lung cancer. New England Journal of Medicine, 389(22), 2063–2075. https://doi.org/10.1056/NEJMoa2307980
Chandana, S. R., Choudury, N. J., Dowlati, A., et al. (2004). First-in-human study of ABBV-706, a seizure-related homolog protein 6 (SEZ6)–targeting antibody-drug conjugate (ADC), in patients (pts) with advanced solid tumors. Journal Clinical Oncology, 42, 16. https://doi.org/10.1200/JCO.2024.42.16_suppl.300
Oronsky, B., Takahashi, L., Gordon, R., Cabrales, P., Caroen, S., & Reid, T. (2023). RRx-001: A chimeric triple action NLRP3 inhibitor, Nrf2 inducer, and nitric oxide superagonist. Frontiers in Oncology, 29(13), 1204143. https://doi.org/10.3389/fonc.2023.1204143
Jayabalan, N., Oronsky, B., Cabrales, P., et al. (2023). A review of RRx-001: A late-stage multiindication inhibitor of NLRP3 activation and chronic inflammation. Drugs, 83(5), 389–402. https://doi.org/10.1007/s40265-023-01838-z
Zhao, H., Ning, S., Scicinski, J., Oronsky, B., Knox, S. J., & Peehl, D. M. (2015). Epigenetic effects of RRx001: A possible unifying mechanism of anticancer activity. Oncotarget, 6(41), 43172–43181. https://doi.org/10.18632/oncotarget.6526
Morgensztern, D., Rose, M., Waqar, S. N., et al. (2019). RRx-001 followed by platinum plus etoposide in patients with previously treated small-cell lung cancer. British Journal of Cancer, 121(3), 211–217. https://doi.org/10.1038/s41416-019-0504-8
Reid, T., Oronsky, B., Scicinski, J., et al. (2015). Safety and activity of RRx-001 in patients with advanced cancer: A first-in-human, open-label, dose-escalation phase 1 study. Lancet Oncol, 16(9), 1133–1142. https://doi.org/10.1016/S1470-2045(15)00089-3
Bonomi, M., Blakaj, D. M., Kabarriti, R., et al. (2023). PREVLAR: Phase 2 a randomized trial to assess the safety and efficacy of RRx-001 in the attenuation of oral mucositis in patients receiving head and neck chemoradiotherapy. International Journal of Radiation Oncology*Biology*Physics, 116(3), 551–559. https://doi.org/10.1016/j.ijrobp.2022.12.031
Johnson M, Awad M, Koyama T, et al. (2023, November). Ifinatamab deruxtecan (I-DXd; DS-7300) in patients with refractory SCLC: A subgroup analysis of a phase 1/2 study. In 2023 World Conference on Lung Cancer. OA05 antibody drug conjugates: The next tsunami, sunday, September 10, 2023 - 15:00 - 16:00| Vol. 18, Issue 11. Supplement, S54–S55.
Oronsky, B., Caroen, S., Oronsky, A., et al. (2017). Electrolyte disorders with platinum-based chemotherapy: Mechanisms, manifestations and management. Cancer Chemotherapy and Pharmacology, 80(5), 895–907. https://doi.org/10.1007/s00280-017-3392-8
Rottenberg, S., Disler, C., & Perego, P. (2021). The rediscovery of platinum-based cancer therapy. Nature Reviews Cancer, 21(1), 37–50. https://doi.org/10.1038/s41568-020-00308-y
Oronsky, B., Ray, C. M., Spira, A. I., Trepel, J. B., Carter, C. A., & Cottrill, H. M. (2017). A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Medical Oncology, 34(6), 103. https://doi.org/10.1007/s12032-017-0960-z
Herzog, B. H., Devarakonda, S., & Govindan, R. (2021). Overcoming chemotherapy resistance in SCLC. Journal of Thoracic Oncology, 16(12), 2002–2015. https://doi.org/10.1016/j.jtho.2021.07.018
Lissa, D., Takahashi, N., Desai, P., et al. (2022). Heterogeneity of neuroendocrine transcriptional states in metastatic small cell lung cancers and patient-derived models. Nature Communications, 13(1), 2023. https://doi.org/10.1038/s41467-022-29517-9
Acknowledgements
The authors gratefully and humbly acknowledge SCLC patients for their often altruistic willingness to enroll on clinical trials to help others, not necessarily themselves, with the disease. Additional thanks to Dr. Christopher Larson for his oversight of the manuscript.
Funding
Open Access funding provided by EpicentRx Inc.
Author information
Authors and Affiliations
Contributions
BO and TR: Conceptualization, figures, writing—original draft, review, and editing. LM, YS, XW, LZ, and NA: Conceptualization, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
BO, SC, TR are employed by EpicentRx LM, YS, XW, LZ, and NA are employed by SciClone Pharmaceuticals NA has no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Oronsky, B., Abrouk, N., Mao, L. et al. Lost at SCLC: a review of potential platinum sensitizers. Cancer Metastasis Rev (2024). https://doi.org/10.1007/s10555-024-10207-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10555-024-10207-5