Abstract
Aim
To assess in a phantom and in a clinical study the influence of different reconstruction parameters on quantitative SPECT/CT values in the assessment of cardiac transthyretin amyloidosis (ATTR-CA).
Method
A hybrid SPECT/CT camera with a proprietary software for SPECT/CT-based quantification of myocardial uptake of 99mTc-DPD was used. Images were reconstructed with 6 different protocols, differing in iterations, subset and Gaussian filter. Quantitative values were tested in phantom and clinical studies across different reconstruction protocols. Values were automatically calculated both as kBq/ml and as maximum, mean and peak standardized uptake value (SUV).
Results
The standard parameters provided by the manufacturer (reconstruction 1) yielded higher accuracy in quantifying, with measuring 97.1% of the expected activity in the phantom. Reconstructions with higher Gaussian filter caused a systematic underestimation of quantified values of 27.2% (p < 0.01). Results were replicated in the clinical study consisting of 155 patients with suspected ATTR-CA, wherein changing the number of iterations and subsets was not associated with a statistically significant difference in quantitative values compared to reconstruction 1, while a higher Gaussian filter caused inaccurate quantification with up to 24% of difference measured.
Conclusion
Different reconstruction parameters can impact quantitative values on 99mTc-DPD SPECT/CT. Therefore, parameters should be maintained consistently across different acquisitions and different centres.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For the diagnosis of transthyretin cardiac amyloidosis (ATTR-CA), the visual interpretation of 99mTc-3,3-diphosphono-1,2 propanodicarboxylicacid (99mTc-DPD) scintigraphy is the non-invasive diagnostic standard [1]. However, recent studies have showed the advantage of quantitative single photon emission computer tomography (SPECT/CT) both in the diagnosis [2, 3] and in the assessment of therapy response [4]. Moreover, novel insights indicate that the Perugini score, a visual interpretation score, is not adequate to assess the degree of the disease and the rapidity of its evolution [5, 6]. Therefore, the focus will be on the quantitative data. SPECT/CT quantification can be performed using a commercially available software [7], which allows to calculate standardized uptake values (SUV) of the heart. Of note, such calculation follows image reconstruction with standard parameters, which are generally provided by the manufacturer.
In this regard, it should be noted that prior studies featuring positron emission tomography (PET/CT) reported that different reconstruction parameters can influence the accuracy of SUV measurements in clinical settings [8]. Furthermore, phantom studies also showed the influence of reconstruction parameters on quantative SPECT/CT [9]. To date, no studies specifically focused on the impact of reconstruction parameters on quantative SPECT/CT for the assessment of ATTR-CA. Hence, to address this knowledge gap, the aim was to assess in a phantom and in a clinical study of suspected ATTR-CA patients, the influence of different reconstruction parameters on SPECT-based SUV.
Methods
Phantom study
A phantom study was performed using the NEMA IEC body phantom [10]. This was filled with a solution of 99mTc-O4 and water, in order to obtain an activity concentration in the sphere with a factor of 4 higher than the background. By knowing the exact volume and activity in both the sphere and phantom the activity concentration for the sphere and background were calculated.
Patients
A total of 171 patients with suspected ATTR-CA were scanned between September 2018 and March 2023 at the Department of Nuclear Medicine, Inselspital, Bern University Hospital. All have been registered in the database of the Swiss-CARE registry, NCT04776824. Sixteen patients were excluded from the retrospective analysis due to inappropriate study quality and/or excessive patient movement. The reported investigations were carried out in accordance with the principles of the Declaration of Helsinki. Signed informed consent was obtained by all participants.
Acquisition
The acquisition protocol is based on a previous paper on quantative cardiac SPECT/CT [2] with slight modifications. In detail, patients underwent an intravenous injection of 674.19±10.25 MBq of Tc99m-DPD. Residual activity in the syringe was measured and indicated in the software for the acquisition. Three hours after injection, patients were acquired on a hybrid double-headed SPECT/CT system (Symbia Intevo Bold™, Siemens Healthineers, Hoffman Estates, IL, USA), equipped with low-energy, high-resolution collimators. Acquisition parameters are displayed in the supplemental Table 1. First, the patients underwent a planar acquisition of the whole body (energy set on 140 keV with a 20% window, 15 cm/min). Right after, a SPECT/CT of the thorax was performed (180° configuration, 32 steps, 25s/step, Supplemental Table 1). A low-dose CT was performed for attenuation correction, anatomical localization and to allow for the quantification of DPD-uptake. CT parameters are also displayed in the supplemental Table 1.
Reconstruction
Both for phantom and clinical images, data were reconstructed to a 256 × 256 matrix using a proprietary software (Symbia Intevo Bold™ with xSPECT Quant™). Six different combinations of parameters were used, as displayed in Table 1, each with differences in the number of iterations, number of subsets and 3D Gaussian full-width half-maximum (FWHM) filters. The updates, iterations times subsets, was kept constant to test if quantative outcomes would change over different combinations of iterations and subset at constant updates. Other reconstruction parameters were kept the same across the sets of images, as they could not be modified without affecting the possibility to quantitatively reconstruct the images. Attenuation, scatter corrections and resolution recovery were automatically applied as of standard in using this quantification software. The attenuation correction is performed using a voxel-based forward/back projector with the linear attenuation coefficient generated by a bi-linear conversion based on fit to reference material derived from the CT. Whereas the scatter correction is conducted using an additive in forward projector scatter compensation from energy window-based scatter projection estimates. And the resolution recovery is employing a forward/back projector with depth-dependent adjustments based on measured ROR [7]. Representing the clinical setting.
For quantitative SPECT/CT the calibration factor (CF) is an import part. The CF of the used SPECT camera is determined half yearly. This is done using the National Institute of Standards and Technology (NIST)-traceable approach, here a calibrated sensitivity source (CSS) is utilized to calibrate the scanner and cross-check the dosimeter accuracy using the same source [11].
Data analysis
Images were visually and semi quantitatively evaluating using appropriate workstation (Syngo.Via, Siemens Healthineers, Erlangen, Germany). For the phantom study, a standard volume of interest (VOI) was manually drawn around the ‘hot’ sphere and on the background across all 6 different reconstructions, maximum and mean activity was automatically calculated within each VOI in kBq/ml for each reconstruction. For the clinical study, quantitative data within the myocardium were assessed by generating an isocontour VOI with 40% threshold of peak activity around the myocardial wall. Consistent with previous reporting [2], cardiac VOIs were manually adjusted to fit cardiac shape based on CT contouring if no detectable uptake was present. Maximum, mean and peak values were automatically calculated both as kBq/ml and as standardized uptake values (SUVmax, SUVmean and SUVpeak, respectively). Values were also normalized to bone activity. To that end, another isocontour VOI with 40% threshold of peak activity was drawn around a thoracic vertebra, after visual verification on CT images of the absence of relevant alterations. Then, normalized SUVs were obtained by dividing the respective value of myocardial and bone uptake (nSUVmax, nSUVmean, nSUVpeak, respectively).
Statistical analysis
The statistical analysis was done using R (version 4.3.1, The R Foundation for Statistical Computing). Variables are presented as mean ± standard deviation (SD) for continuous variables and as number (%) for categorical data. Values across different reconstructions were compared to the Reconstruction protocol with standard parameters (i.e. Reconstruction 1) pairwise by means of Student T-test. A p-value less then 0.05 was considered to show a difference of statistical significance.
Results
Phantom study
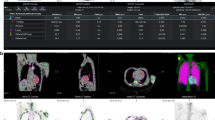
The calculated, expected activity concentrations in the phantom were 47.8 kBq/ml in the sphere and 11.9 kBq/ml in the background, with a resultant ratio of 4.02. Table 2 shows the values of the activity in the sphere and in the background across different reconstructions and for the sphere and background mean the percentage difference to the calculated values. The values from Reconstruction 1 were closest to the expected activity both in the sphere and in the background in ratio (46.43 kBq/ml and 11.02 kBq/ml, respectively, ratio 4.21). The sphere mean of Reconstruction 1 had the smallest percentual difference to the calculated values. In Figure 1 a visual representation of the 6 different reconstructions on the sphere is shown.
Representative image showing the six different reconstructions in the phantom study Reconstructions are numbered to fit the respective Reconstruction in Table 1
Patient study
The final study population consisted of 155 patients, whose main data are displayed in Table 3. The majority of the patients were male (86.5%) and 105 out of 155 (67.7%) had a final diagnosis of ATTR-CA, according to Perugini grade 2 or 3 for 98 patients. In the other 7 patients, ATTR-CA was confirmed in 2 due to evidence of increased extracellular volume on a subsequently performed cardiac magnetic resonance, in 4 due to evidence of increased DPD-uptake in the basal segments of left ventricle on SPECT/CT images and in one as SUVmax was higher than normal values reported in literature.
Tables 4 and 5 show the differences in calculated activity (kBq/ml) as well as SUVs across the 6 reconstruction protocols. Based on the results of the phantom study, it was assumed that Reconstruction 1 would provide the highest accuracy in quantification for the clinical study as well. Therefore, the results obtained with the other reconstruction parameters were compared to that obtained in Reconstruction 1. No differences in any considered variable was seen among Reconstruction 1, 2, and 3, meaning that changing the number of iterations and subsets was not associated with a statistically significant change in calculated activities and/or SUVs, both in the myocardium and in the bone. Of note, no differences were seen also with Reconstruction 5, meaning that a lower Gaussian filter is also unlikely to modify the quantification.
Conversely, applying a higher Gaussian filter (reconstruction 4 and 6) had a significant impact on calculated activities and SUVs. While the impact was less pronounced for SUVmean within the heart (p > 0.05), a significant difference could be seen both for SUVmax of the myocardium and for SUVmax and SUVmean of the bone (p < 0.05). Of note, the effect on to-bone normalized values is not significant, considering the fact that both heart and bone uptake are affected similarly from this incorrect calculation. The visual difference between the 6 reconstruction protocols in the myocardium in one patient can be seen in Fig. 2.
Discussion
Quantification in 99mTc-DPD SPECT/CT in the assessment of ATTR-CA is gaining interest for its possible clinical applications, therefore it is required, that calculated values are consistent across acquisitions and that they are representative of the real myocardial activity.
In our study, both in the phantom and in patients, changing the number of iterations and subsets had a limited impact on the calculated data, but differences became significant if a higher Gaussian filter was applied. Emphasising the effect of the Gaussian filter on the quantative analysis of 99mTc-DPD SPECT/CT, disregarding the potential effects on visual image quality. Interestingly, even if image quality might be similar, the results in terms of quantification are not the same even if the updates are the same. The reason might be that high iterations number render the image noisier with a gain in resolution and contrast.
Representative images showing the different reconstructions. An axial SPECT slice is displayed. To note, images are more noisy and smoother in reconstruction 4 and 6, being those with a higher Gaussian filter. In the other reconstructions, qualitative evaluation does not show significant differences across the reconstructions
Considering the differences across different reconstructions, an important question arises: what is the combination of parameters which yields highest accuracy in quantifying the activity within a defined structure? As somewhat expected, the results on our phantom study suggest that that highest accuracy is yielded with Reconstruction 1, which is also evident considering the sphere-to-background ratio. On the other hand, the relatively low background activity probably explains the consistency of measured background values across different reconstructions. Of note, the quantification software we used in our study is commercially broadly available and studies have showed the accuracy of measurements across different institutes, provided the same reconstruction protocol is used [15].
Considering the phantom study, it could be advised that the parameters of Reconstruction 1 are more likely to prove accurate in clinical studies. The subsequent clinical studies showed that modifying the iterations and subset only, does not cause statistically different values. This is again in accordance with prior studies in the influence of Gaussian filter on SUV values [10], but it should be noted that the lack of a statistically significant difference among semiquantitative values does not per se translate into a nonsignificant difference from a clinical point of view.
Previous studies have showed the clinical relevance of using quantification in the assessment of ATTR-CA [2, 3], and recent studies also showed a decrease in DPD-uptake after therapy with TTR-stabilizer therapy [4]. Considering the clinical importance in the observation of a decrease in DPD uptake after therapy, there is the need for a perfect reproducibility of the results in subsequent SPECT/CT examinations. As such, our study emphasize the importance of consistent reconstructions parameters across examinations, to ensure intra-patient comparability of SUV values.
To date, no national or international guidelines have been published on quantitative SPECT/CT in ATTR-CA. Considering the importance of quantitative SPECT/CT in the diagnosis and in the evaluation of the response to therapy, such publication would be helpful for the nuclear cardiology community, and our paper represents a fundamental step in its drafting.
Some limitations in our study should be acknowledged. First, we made a small selection of different combinations among iterations, subsets and Gaussian filters, being the only parameters which can be modified when using this quantification software. As such, our results are partial, but we could confirm the different impact of different parameters on quantified values. In this regard, we did not test filters other than Gaussian, according to the technical paper provided with xSPECT Quant software [7]. The fact that results differ when using a high filter gives reliance that further modifications would only amplify those differences. As such, there is the need for keeping filters as constant as possible across examinations and centres.
Second, the results of our study are based on a single, proprietary hardware-software system allowing for 99mTc-labelled tracers quantification, and the consistence of quantitative SPECT/CT using different reconstruction parameters across different software and/or camera systems in the assessment of ATTR-CA still need to be elucidated. While the latter falls outside the scope of the present study, our results represent a fundamental basis for further research in this regard.
Furthermore, the used protocol in which the number of updates was equal across the different reconstruction might be unconventional as earlies papers highlighted the effect of increasing the number if iterations [14]. The hypothesis was that quantitative values might change with varying iterations and subsets, while keeping the updates constant. This was done in the first three reconstructions; in the remaining three, the iterations and subsets were not changed to solely observe the effect of the filter. Of note, values differed among the different reconstructions, although not reaching a statistical significance. This reinforces the concept that not only the number of updates, but also the differences in the number of subsets/iterations across a fix number of updates might cause differences in the quantification of SPECT performed for the assessment of ATTR-cardiomyopathy.
Conclusion
For the first time, the impact of different reconstruction parameters in a phantom and clinical study using quantitative 99mTc-DPD SPECT/CT was investigated. This adds another tesserae in the technical requirements needed to yield consistent quantitative values in the assessment of patients with suspected or diagnosed ATTR-CA.
In the assessment of ATTR-CA with quantitative 99mTc-DPD SPECT/CT, different reconstruction parameters can impact calculated activities and/or SUVs. While the number of iterations and subsets play only a marginal role in this regard, a high Gaussian filter can cause a systematic underestimation of such values. Hence, acquisition parameters should be kept identical across different acquisitions and/or centres.
Data availability
No datasets were generated or analysed during the current study.
References
Hutt DF et al (2017) Dec., ‘Prognostic utility of the Perugini grading of 99mTc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid’, European Heart Journal - Cardiovascular Imaging, vol. 18, no. 12, pp. 1344–1350, https://doi.org/10.1093/ehjci/jew325
Caobelli F, Braun M, Haaf P, Wild D, Zellweger MJ (2020) ‘Quantitative 99mTc-DPD SPECT/CT in patients with suspected ATTR cardiac amyloidosis: Feasibility and correlation with visual scores’, J. Nucl. Cardiol., vol. 27, no. 5, pp. 1456–1463, Oct. https://doi.org/10.1007/s12350-019-01893-8
Scully PR et al (2020) Jun., ‘DPD Quantification in Cardiac Amyloidosis: A Novel Imaging Biomarker’, Jacc. Cardiovascular Imaging, vol. 13, no. 6, p. 1353, https://doi.org/10.1016/j.jcmg.2020.03.020
Papathanasiou M et al (2023) Jul., ‘Regression of Myocardial 99m Tc-DPD Uptake After Tafamidis Treatment of Cardiac Transthyretin Amyloidosis’, J Nucl Med, vol. 64, no. 7, pp. 1083–1086, https://doi.org/10.2967/jnumed.122.265352
Caobelli F, Rischpler C (Jul. 2024) Collection on molecular imaging in cardiac amyloidosis. Eur J Nucl Med Mol Imaging 51(8):2163–2164. https://doi.org/10.1007/s00259-024-06739-1
Caobelli F et al (2024) Jun., ‘Prognostic Value of [99mTc]Tc-DPD Quantitative SPECT/CT in Patients with Suspected and Confirmed Amyloid Transthyretin–Related Cardiomyopathy and Preserved Left Ventricular Function’, Journal of Nuclear Medicine, vol. 65, no. 6, pp. 944–951, https://doi.org/10.2967/jnumed.123.266926
Vija A, Hans (2013) ‘Introduction to xSPECT*Technology: Evolving Multi-modal SPECT to Become Context-based and Quantitative’, Siemens Medical Solutions USA, Inc., Molecular Imaging, White Paper, Accessed: Sep. 09, 2023. [Online]. Available: https://cdn0.scrvt.com/39b415fb07de4d9656c7b516d8e2d907/1800000003359764/038bd47eb17e/MI-2706_xSPECT_TECHNICAL_White-paper_final_1800000003359764.pdf
Adams MC, Turkington TG, Wilson JM, Wong TZ (2010) ‘A Systematic Review of the Factors Affecting Accuracy of SUV Measurements’, American Journal of Roentgenology, vol. 195, no. 2, pp. 310–320, Aug. https://doi.org/10.2214/AJR.10.4923
Halim F, Yahya H, Jaafar KN, Mansor S (2021) ‘Accuracy Assessment of SUV Measurements in SPECT/CT: A Phantom Study’, J. Nucl. Med. Technol., vol. 49, no. 3, pp. 250–255, Sep. https://doi.org/10.2967/jnmt.120.259168
Tsutsui Y, Awamoto S, Himuro K, Umezu Y, Baba S, Sasaki M (2018) Characteristics of smoothing filters to achieve the Guideline recommended Positron Emission Tomography Image without Harmonization. Asia Ocean J Nucl Med Biol 6(1):15–23. https://doi.org/10.22038/aojnmb.2017.26684.1186
Vija AH, von Gall C, Ghosh P (2018) ‘Accurate, reproducible, and standardized quantification’, Siemens Medical Solutions USA, Inc., Molecular Imaging, White Paper, Accessed: Jul. 15, 2024. [Online]. Available: https://marketing.webassets.siemens-healthineers.com/1800000004691530/f7401b228654/MI-3610_xSPECT_QUANT_WP.FINAL_1800000004691530.pdf
Chen E-J, Safwan Selvam HS, Tan TH, Chew MT (Nov. 2021) Quantitative analysis of xQuant reconstruction algorithm in SPECT/CT. Radiat Phys Chem 188:109683. https://doi.org/10.1016/j.radphyschem.2021.109683
Tomita Y, Ichikawa Y, Hashizume K, Sakuma H (2023) ‘Effect of Gaussian Smoothing Filter Size for CT-Based Attenuation Correction on Quantitative Assessment of Bone SPECT/CT: A Phantom Study’, J Digit Imaging, vol. 36, no. 5, pp. 2313–2321, Jun. https://doi.org/10.1007/s10278-023-00864-3
Kupitz D et al (Dec. 2021) Optimization of SPECT/CT imaging protocols for quantitative and qualitative 99mTc SPECT. EJNMMI Phys 8(1). https://doi.org/10.1186/s40658-021-00405-3
Okuda K et al (2021) Jun., ‘Multicenter Study of Quantitative SPECT: Reproducibility of 99m Tc Quantitation Using a Conjugated-Gradient Minimization Reconstruction Algorithm’, J. Nucl. Med. Technol., vol. 49, no. 2, pp. 138–142, https://doi.org/10.2967/jnmt.120.256131
Funding
The Swiss-CARE registry is supported by funding from Pfizer.
Author information
Authors and Affiliations
Contributions
R.S. and F.C. wrote manuscript; R.S. and F.C. conceived original idea; R.S. and F.C. led the overall study; R.S. performed the reconstructions; N.G. draw the VOI’s; R.S. and K.Z. performed the phantom scans; R.S. performed the statistical analyses; C.M.B., A.A.-O., A.R. and C.G. provided review and editing support. All authors have read and agreed to the published version ofthe manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Disclosures
Robin Schepers has received speaker honoraria from Siemens. Christoph Gräni receives funding from the Swiss National Science foundation, InnoSuisse, CAIM foundation, GAMBIT foundation and Novartis biomedical research foundation, outside of the submitted work. Axel Rominger has received research support and speaker honoraria from Siemens. Federico Caobelli is currently supported by a research grant by Siemens Healthineers and receives speakers Honoraria by Bracco AG and Pfizer AG for matters not related to the present manuscript. All other authors have no competing interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schepers, R., Gözlügöl, N., Zeimpekis, K. et al. The impact of different reconstruction parameters on quantitative 99mTc-DPD SPECT/CT values in the assessment of cardiac transthyretin amyloidosis. Int J Cardiovasc Imaging (2024). https://doi.org/10.1007/s10554-024-03231-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10554-024-03231-7