Abstract
The benefit of repeat assessment of left ventricular (LV) systolic and diastolic function in heart failure (HF) remains uncertain. We assessed the prognostic value of repeat echocardiographic assessment of LV filling pressure (LVFP) and its interaction with cardiac index (CI) in ambulatory patients with chronic HF and reduced ejection fraction (HFrEF). We enrolled 357 patients (age 68 ± 11 years; 22% female) with chronic HFrEF. Patients underwent a clinical and echocardiographic examination at baseline. LVFP as assessed by the 2016 Guidelines and Doppler-derived CI were estimated. After the second echocardiographic examination, patients were followed for a median time of 30 months. The study endpoint included all-cause death and hospitalization for worsening HF. Patients who normalized LVFP or showed persistently normal LVFP at the follow-up examination had a significantly lower mortality rate than those with worsening or persistently raised LVFP (p < 0.0001). After stratification by CI, patients with elevated LVFP and CI < 2.0 l/min/m2 had a further worse outcome than those with elevated LVFP and CI ≥ 2.0 l/min/m2 (p < 0.0001). Multivariate survival analysis confirmed an independent prognostic impact of changes in LVFP, incremental to that of established clinical, laboratory and echocardiographic predictors. Repeat assessment of LVFP and CI significantly improved risk stratification of chronic HFrEF outpatients compared to baseline evaluation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Management of heart failure (HF), improvement of survival and prevention of hospitalizations largely depend on LV structural and/or functional cardiac abnormalities, resulting in a reduced cardiac output (CO) and/or elevated intracardiac pressures at rest or during stress. Currently, Doppler echocardiography can provide reliable and reproducible LV filling pressure measures (LVFP) and forward flow, i.e., stroke volume and CO [1].
Several echocardiographic studies have shown that markers of elevated LVFP have been associated with an adverse prognosis [2], but their reversal toward normality with treatment favourably impact clinical outcome [3,4,5]. The EAEVI Euro-Filling Study has demonstrated that the 2016 ASE/EACVI algorithms are reliable and clinically useful for noninvasively estimating LVFP [6]. Still, the prognostic impact of the temporal changes of LVFP as assessed by the 2016 Guidelines from baseline to follow-up remains unknown. Moreover, it became apparent that diastolic dysfunction (DD) could affect CO [7, 8] and a decreased forward flow is negatively associated with prognosis [9, 10]. Therefore, the present study was designed to ascertain: (1) the prognosis of ambulatory HF patients with persistent or worsening DD, (2) whether the recovery of DD during follow-up was associated with improved patients’ outcome; (3) the prognostic impact of reduced forward flow in patients with persistent or worsening DD at follow-up.
Patients and methods
Study patients
This observational study included consecutive patients with chronic HF referred to our outpatient clinics in Pisa for clinical evaluation and follow-up from 2010 to 2019. The inclusion criteria comprised LV EF ≤ 50% and LV end-diastolic volume (EDV) index > 75 ml/m2.
Exclusion criteria were: recent myocardial infarction or unstable angina (< 3 months), coronary artery bypass graft or percutaneous coronary angioplasty (< 3 months), more than mild aortic or organic mitral valve disease, hypertrophic cardiomyopathy, amyloidosis, active myocarditis, prosthetic valve dysfunction, and inadequate image quality. Patients with atrial fibrillation were not excluded from the study. The study was approved by the local institutional review board. All patients gave written informed consent with their approval to participate in the study. The study was conducted in accordance with the institutional policies, national legal requirements, and the revised Helsinki declaration.
Clinical evaluation
The patient’s functional status was determined according to the New York Heart Association (NYHA) classification. Blood was drawn for natriuretic peptides (NP), B-type natriuretic peptide (BNP) and amino-terminal proB-type natriuretic peptide (NT-proBNP) assays, and measure of renal function. Blood pressure, heart rate and rhythm were recorded. The estimated glomerular filtration rate was calculated from the simplified formula derived from the Modification of Diet in Renal Disease (MDRD) study.
Echocardiographic examination
Transthoracic two-dimensional and Doppler echocardiographic examination (including tissue Doppler) was carried out with an iE33 X5-matrix and an EPIQ 7 Ultrasound instrument (Philips, Andover, Massachusetts) equipped with a 3.5-MHz transducer. The following standard echocardiographic variables were obtained: LV volumes and EF and left atrial volume index (LAVi), which were assessed from apical two- and four-chamber views using the biplane Simpson’s method. From mitral velocity tracings, peak early (E) and late (A) transmitral flow velocities, their ratio E/A, and E wave deceleration time (EDT) were measured from spectral Doppler recordings of LV filling. Doppler tissue imaging longitudinal velocities were recorded with the sample volume placed at the junction between the septal and lateral LV wall and the mitral annulus in the 4-chamber view. A ratio of E/averaged myocardial early velocity of the lateral and septal sites (averaged E/e′) was calculated. Tricuspid annulus plane systolic excursion (TAPSE) was measured from M-mode recordings. Retrograde blood flow of tricuspid regurgitation was recorded using continuous-wave Doppler for measuring peak tricuspid regurgitation velocity (TRV). The peak velocity was assigned as the average of five tricuspid regurgitation envelopes with the greatest maximal velocities and spectral density. The ASE-EACVI 2016 recommendations were used to estimate normal or increased LVFP [11] The LV outflow tract (LVOT) anteroposterior diameter was measured in the parasternal long-axis view, and the LVOT area was estimated as π(d/2)2 (cm2). LV stroke volume was calculated as the product of the LVOT area and the velocity–time integral of the forward flow. LVOT velocity–time integral was measured by tracing the outer edge of the densest (or brightest) portion of the spectral tracing with the pulsed wave Doppler sample volume positioned in the middle of the LVOT below the aortic cusps from the apical 5-chamber or long-axis view, and CO was calculated as stroke volume multiplied by heart rate [12]. Cardiac index (CI) was estimated by dividing CO by body surface area. For each Doppler-based measurement, estimates were obtained from 3 cardiac cycles in sinus rhythm or 5 in patients with atrial fibrillation. Mitral regurgitation severity was graded using the vena contracta method or by measuring the effective regurgitant orifice area into mild, moderate and severe.
Echocardiographic hemodynamic classification
Study patients were classified according to the presence or absence of DD in the light of ASE-EACVI 2016 recommendations. Patients with elevated LVFP were considered those with E/A ≥ 2.0 or exhibiting > 50% of the following criteria: an average (of septal and lateral) E/e′ > 14, a septal e′ < 7 cm/c or lateral < 10 cm/s, a TRV > 2.8 m/s and a LAVi > 34 ml/m2. LVFP was considered normal if < 50% of previous criteria were present or when E/A ≤ 0.8 or E ≤ 50 cm/s. When ASE-EACVI 2016 recommendations could not determine DD, identification of elevated LVFP relied on an algorithm based on noninvasively measured pulmonary diastolic pressure [13]. Patients were defined as having normal forward flow if they had CI ≥ 2.0 l/min/m2, whilst a reduced forward flow was characterized by CI < 2.0 l/min/m2 [9].
Follow up
Patients were evaluated at baseline (index echocardiogram) and underwent clinical evaluation, BNP or NT-proBNP assessment and repeat echocardiographic examinations 6 ± 3 months afterwards. At follow-up, patients were classified into four groups based on changes in LV diastolic function: (1) persistently normal, if the diastolic function was normal on both baseline and follow-up examinations; (2) reversible, if DD was present only at baseline; (3) worsening, if DD was present only at follow-up; (4) persistently abnormal, if the diastolic function was abnormal on both baseline and follow-up examinations. Abnormal natriuretic peptide concentration at follow-up was defined as BNP ≥150 pg/ml or NT-proBNP ≥450 pg/ml, along with failure to demonstrate ≥30% reduction from baseline [14].
Study endpoints
The endpoint was a composite of all-cause mortality and HF hospitalization. Survival data were obtained through follow-up visits of patients or, in the case of missed visits, through telephone contacts. Follow-up data were obtained by reviewing the patient’s hospital records; death certificates were obtained in case of need. Survival analyses were performed considering the day of the follow-up examination as the starting day. For patients without events, the date of the last contact was considered the end of follow-up for survival analysis.
Statistical analysis
Data for continuous variables were presented as mean ± SD or as median with interquartile range in case of non-normal distribution. Categorical variables were presented as numbers and percentages. Differences in continuous variables among groups were analyzed by the Student’s t-test or ANOVA, as appropriate. For non-parametric variables, the Mann–Whitney U test and the Kruskal–Wallis test were used. Categorical variables were compared by the Chi-square test or Fisher exact probability test when indicated. Two-way repeated-measures ANOVA was used to assess the effect of LV changes in LV diastolic function on CI. Kaplan–Meier curves were plotted to assess event-free survival probability across groups. Multivariate Cox proportional hazards regression was used to estimate the prognostic value of changes in LV diastolic function. The following variables were tested in the analysis: age, male gender, atrial fibrillation, diabetes mellitus, coronary artery disease, glomerular filtration rate, NYHA class, abnormal natriuretic peptide, ejection fraction, cardiac index < 2.0 l/min/m2, TAPSE, mitral regurgitation effective regurgitant orifice area, and LV diastolic dysfunction at follow-up. Model discrimination was explored by receiver operating characteristic (ROC) analysis using the linear predictor score obtained from the proportional hazards model. The likelihood ratio test was considered to compare the performance among nested models and assess the incremental value of LV diastolic function changes over established predictors of outcome. Data were analyzed using the IBM SPSS Statistics software, v. 24. Differences were considered statistically significant for P < 0.05.
Results
The study population included 357 patients (mean age 68 ± 11 years; 22% female). Table 1 shows the main characteristic of the study patients. 44% of patients had ischemic LV dysfunction, 31% were in NYHA class I, 49% in NYHA class II, and 20% in NYHA class III. According to the study design, all patients had LV systolic dysfunction (mean LV EF 33 ± 7, range 11–50%); 31% had severe LV dysfunction, defined as EF < 30%.
Among patients with ischemic LV dysfunction, 47% underwent percutaneous coronary angioplasty, and 16% were submitted to coronary bypass grafting. Cardiac resynchronization therapy was performed in 53 of the total study population, whereas an automated defibrillator was implanted in 97.
Of the patients who met the inclusion criteria, feasibility was very high since LV DD could not be determined in 9 either using the criteria as recommended in the 2016 Guidelines or by the algorithm based on noninvasively measured pulmonary diastolic pressure. These patients were subsequently excluded from the analyses. The proportion of patients that relied on the algorithm based on noninvasively measured pulmonary diastolic pressure to identify DD was 14% at baseline and 13% at follow-up.
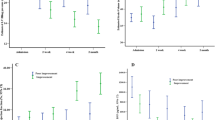
LV DD, assessed separately at baseline or the follow-up examination (Fig. 1), was an independent predictor of outcome, also after stratification by CI (Supplementary Figs. 1–4). The comparison between patients with persistently normal diastolic function, reversible DD, worsening, and persistently abnormal diastolic function is shown in Table 2. Kaplan–Meier curves showed that changes in LV diastolic function from baseline to follow-up examination were strongly associated with the clinical outcome (Fig. 2, p < 0.0001). Patients with worsening or persistently abnormal diastolic function had a significantly higher risk of events than those with persistently normal diastolic function or reversible DD (p < 0.0001 for all pair-wise comparisons). Pooling patients with worsening or persistently abnormal diastolic function and stratifying them according to normal or abnormal CI (Table 3) showed the latter to have a worse outcome than those with normal CI (Fig. 3). Notably, when patients were categorized into those with no DD at baseline, those with DD at baseline but recovered at follow-up, and those with DD at baseline that persisted at follow-up, the last two groups showed opposite changes in CI at follow-up (Fig. 4).
Baseline spectral Doppler mitral flow recordings of the left ventricular filling (A) and tissue Doppler imaging (B) of mitral annulus indicative of elevated left ventricular filling pressure (averaged E/e′ 28.6). Reversal of mitral flow at follow-up (C) after optimized tailored therapy (as tissue Doppler imaging was unchanged, E/e′ had diminished to 13.4)
Patients with (1) persistently normal diastolic function or (2) reversible diastolic dysfunction had significantly better outcome than (3) those with persistenly abnormal diastolic function or (4) worsening diastolic function. Log-rank pair-wise (Mantel–Cox): p < 0.001 for curve 3 vs 1 or 2; p < 0.001 for curve 4 vs 1 or 2, p = 0.76 for curve 1 vs 2; p = 0.78 for curve 3 vs 4
Survival of patients grouped according to (1) persistently normal diastolic function or reversible diastolic dysfunction, (2) persistently abnormal or worsening diastolic function with cardiac index ≥ 2.0 l/min/m2, (3) persistently abnormal or worsening diastolic function with cardiac index < 2.0 l/min/m2. CI cardiac index. Log-rank pair-wise (Mantel–Cox): p < 0.001 for all comparisons
ROC analysis across nested models also showed a progressive increase in the area under the curve considering an initial model including only clinical variables, then adding natriuretic peptides, echocardiographic variables and changes in LV diastolic function (Fig. 5). Cox regression analysis also showed that LV diastolic function changes were associated with the clinical endpoint, independent of confounders (Supplementary Table 1). In particular, persistently abnormal or worsening diastolic function at follow-up was associated with a 4.5-fold higher risk of death or HF hospitalization. Among the other variables that remained independently associated with clinical outcome in multivariate analysis, the strongest predictors were NYHA class, CI < 2.0 l/min/m2 and EF < 30%, whereas age maintained a borderline significance (Supplemental file 4). An alternative model built by considering for EF the best cut-off provided by ROC analysis (AUC 0.82 [0.78–0.87], p < 0.0001; best cut-off < 33%, 76.0% sensitivity, 75.1% specificity) showed similar results. The incremental analysis confirmed that LV diastolic function changes added significant prognostic information to a comprehensive model, including clinical variables, natriuretic peptide, and echocardiographic variables (Supplementary Fig. 5).
Discrimination analysis: Area under the curve of the initial model, including clinical and demographic variables, and the contribution of natriuretic peptides, echocardiographic variables and changes in left ventricular diastolic function at the Receiver Operating Characteristic analysis across nested models. Clinical: AUC 0.79 (0.74–0.84); Clinical + BNP: AUC 0.83 (0.78–0.87); Clinical + BNP + Echo: AUC 0.88 (0.84–0.92); Clinical + BNP + Echo + Peristently abnormal or worsening DD: AUC 0.91 (0.88–0.94). AUC area under the curve, BNP B-type natriuretic peptide, DD diastolic dysfunction
Discussion
Repeat echocardiography, including DD and CI evaluation, helps in risk stratification of chronic HF outpatients. The prognostic impact was worse in patients with either persistently abnormal or worsening diastolic function. The combination with CI improved prognostication, and patients with either DD or compromised CI at follow-up had the worst outcome.
The prognostic value of left ventricular filling pressure and its change over time
Much has been written about the value of reducing LVFP to improve patient’s clinical outcome [15]. Recent data showing lower rates of HF and all-cause hospitalizations during the year after implantation of the CardioMEMS pulmonary artery pressure sensor boosted the role of repeat assessment of LVFP to guide optimum therapy for ambulatory HF patients [16].
Echocardiography has become the most important investigation for HF patients since it provides LV function measures, both systolic and diastolic. Several parameters related to hemodynamic variables, including LVFP, have been recognized and reported. A restrictive LV filling pattern (i.e., a short isovolumic relaxation time, mitral EDT < 150 ms) and an averaged E/e′ ≥ 13 reliably identify elevated filling pressure, particularly in patients with compromised LV EF [17, 18]. Although echocardiography allows serial non-invasive assessment at relatively low cost and excellent accessibility, current HF Guidelines do not recommend periodically repeated echocardiograms in the follow-up of relatively clinically stable patients [19]. However, some available studies have shown that repeated evaluations with Doppler echocardiography can be of prognostic relevance in HF patients. The estimation of LVFP may be accomplished using the 2016 ASE/EACVI recommendations [20]. Still, to the best of our knowledge, no study addressed the predictive value of either elevated LVFP or reversing DD during follow-up in ambulatory HF patients evaluated by the algorithm proposed by the Guidelines.
In our study, the presence of echocardiographic evidence for DD at follow-up, resulting from persistently abnormal or worsening diastolic function, provided incremental prognostic information over and above that given by a comprehensive evaluation of clinical and demographic variables, absolute or relative NP target levels, and other echocardiographic parameters.
The role of compromised forward flow and of its changes over time
Low-output HF represents a hallmark of advanced HF that is not infrequent in patients with chronic HF [21]; however, little information is available on the prognostic value of noninvasively measured CO and related parameters. Since Doppler echocardiography allows for quantitative assessment of forward flow, a comprehensive echocardiographic examination incorporating measurement of stroke volume, CO, and CI could be obtained [22, 23].
An echocardiography hemodynamic classification guided by LVFP and CI baseline measures can be accomplished to estimate the patient’s hemodynamic status and advise on treatment optimization in the acute setting [24]. Therefore, we argued whether an echo-directed categorization of chronic HF patients according to hemodynamic profiles, i.e., no DD, DD with normal CI and DD with reduced CI, can provide detailed information on both baseline conditions and changes that occur over time. In this study, we addressed the prognostic importance of the changes of systolic and diastolic parameters during patients’ follow-up. Patients with either persistently abnormal or worsening diastolic function and reduced CI had the worst prognosis. In contrast, those whose DD recovered during the follow-up demonstrated amelioration of forward flow that likely contributed to the improved clinical status and prognosis. The finding of the increase in CO in patients showing diastolic function recovery is not new but consistent with previous observations that reported an association of improved diastolic function with higher CO in patients with HF, irrespective of LV EF [7]. Nevertheless, our finding was the first to describe it using echocardiography in chronic HF. Several mechanisms may be brought into play to explain the latter result, including an actual increase in myocardial contractility, a decrease in functional mitral regurgitation with a redistribution of intracardiac volumes to supplement the forward flow, or the heart’s ability to increase the forward flow in response to a decrease in LVFP, as most dilated ventricles operate far beyond the level of LVFP at which stroke volume depends on increments of LVFP.
Clinical implications
With advances in outpatient management for relatively stable ambulatory patients, new challenges are now seen, most notably preventing clinical decompensation and cardiac-related death [25,26,27,28,29,30,31]. It has been demonstrated that the recovery of LVFP during clinical follow-up can improve morbidity and mortality in patients with HF [32]. The present study supports the value of repeat echocardiographic evaluation to obtain a simultaneous picture of the patient’s hemodynamic profile and eventually to guide optimum therapy. As a matter of fact, longitudinal assessment of diastolic function as well as forward flow may be useful to monitor the responses to diuretic therapy and evidence-based medications, including iSGLT2 and sacubitril/valsartan, and the contribution of devices and interventions. In contrast with a previous report that comprised only a limited number of echo variables [33,34,35], our study results showed that a comprehensive assessment of repeatedly measured echocardiographic hemodynamic parameters, such as LVFP and CI, may help risk stratification in patients with chronic HF. An interesting finding with practical implications is that the predictive performance of CI in our population was independent of that of EF. This suggests that the additional time needed to calculate CI, which is not part of the routine echocardiographic examination, might be justified in these patients.
Limitations
There are a number of well known limitations in the echocardiographic estimation of LVFP that may sometimes lead to the incorrect classification of DD. In a systematic metanalysis, the clinical reliability of echocardiographic surrogate markers of LVFP across different cardiovascular entities has been evaluated. The maximum bias was reported in patients with HFpEF, while the bias was comparatively less in HFrEF, and an integrated approach of several echocardiographic parameters showed a valuable estimate [36]. A large study that combined multiple measurements as recommended in the 2016 Guidelines has recently shown that echocardiography can reliably identify patients with elevated LVFP with high feasibility and good accuracy [37]. The optimal timing of repeat echocardiography remains uncertain, probably depending on the individual patient’s clinical condition. Estimating stroke volume is prone to measurement errors amplified by extrapolating measures such as LVOT velocity–time integral into hemodynamic variables such as CI. However, the echocardiographic data reported and analyzed in this study were collected according to contemporary quality standards and reflect the data measured in clinical practice that are regularly utilized for decision-making, such as calculations of aortic valve area in patients with aortic stenosis. Although novel classifications of HF by echocardiography have been proposed [38], in the present study, we used a CI of 2.0 l/min/m2 as a cutoff of forward flow based on a hemodynamic study indicating that peripheral hypoperfusion at CI level at or below 2.0 l/min/m2 was associated with an adverse outcome [9].
Conclusions
A comprehensive repeat echocardiographic assessment of LVFP and forward flow has the potential of characterizing the hemodynamic status and prognosis of patients with HFrEF Patients with either normal or recovered diastolic function and preserved CI at follow-up as a result of medical and/or interventional therapy had a more favourable clinical outcome. This study seems to favour the systematic use of a 2-point observation system separated by 3 to 6 months for assessing prognosis.
References
Mele D (2019) From left ventricular ejection fraction to cardiac hemodynamics : role of echocardiography in evaluating patients with heart failure
Lester SJ, Tajik AJ, Nishimura RA et al (2008) Unlocking the mysteries of diastolic function deciphering the rosetta stone 10 years later. J Am Coll Cardiol 51:679–689. https://doi.org/10.1016/j.jacc.2007.09.061
Pozzoli M, Capomolla S, Pinna G et al (1996) Doppler echocardiography reliably predicts pulmonary artery wedge pressure in patients with chronic heart failure with and without mitral regurgitation. J Am Coll Cardiol 27:883–893. https://doi.org/10.1016/0735-1097(95)00553-6
Pinamonti B, Zecchin M, Lenarda ADI et al (1997) Persistence of restrictive left ventricular filling pattern in dilated cardiomyopathy: an ominous prognostic sign. J Am Coll Cardiol 29:604–612. https://doi.org/10.1016/S0735-1097(96)00539-6
Whalley GA, Mhs C, Doughty RN et al (2002) Pseudonormal mitral filling pattern predicts hospital re-admission in patients with congestive heart failure. J Am Coll Cardiol 39:1787–1795. https://doi.org/10.1016/S0735-1097(02)01868-5
Lancellotti P, Galderisi M, Edvardsen T et al (2017) Echo-Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro-Filling study. Eur Heart J. https://doi.org/10.1093/ehjci/jex067
Tobushi T, Nakano M, Hosokawa K et al (2017) Improved diastolic function is associated with higher cardiac output in patients with heart failure irrespective of left ventricular ejection fraction. J Am Heart Assoc 6(3):e003389. https://doi.org/10.1161/JAHA.116.003389
Stevenson LW, Tillisch JH (1986) Maintenance of cardiac output with normal filling pressures in patients with dilated heart failure. Circulation 74:1303–1308. https://doi.org/10.1161/01.CIR.74.6.1303
Cooper LB, Mentz RJ, Stevens SR et al (2016) Hemodynamic predictors of heart failure morbidity and mortality: fluid or flow? J Cardiol Fail 22:182–189. https://doi.org/10.1016/j.cardfail.2015.11.012
Hamdan R, Charif F, Zein A, Issa M, Najjar C, Abdallah H, Fakih SSM (2019) Noninvasive monitoring of cardiac output: a useful tool yet? J Cardiovasc Echogr 29:165
Nagueh SF, Smiseth OA, Appleton CP et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 29:277–314. https://doi.org/10.1016/j.echo.2016.01.011
Hahn RT, Pibarot P (2017) Accurate measurement of left ventricular outflow tract diameter: comment on the updated recommendations for the echocardiographic assessment of aortic valve stenosis. J Am Soc Echocardiogr 30:1038–1041. https://doi.org/10.1016/j.echo.2017.06.002
Barbier P, Cucco C, Guglielmo M et al (2020) Estimation of increased pulmonary wedge pressure by an algorithm based on noninvasively measured pulmonary diastolic pressure in cardiac patients independent of left ventricular ejection fraction. Echocardiography 37:215–222. https://doi.org/10.1111/echo.14581
Ibrahim NE, Burnett JC, Butler J et al (2020) Natriuretic peptides as inclusion criteria in clinical trials: a JACC: heart failure position paper. JACC Heart Fail 8:347–358. https://doi.org/10.1016/j.jchf.2019.12.010
Stevenson LW (1999) Tailored therapy to hemodynamic goals for advanced heart failure. Eur J Heart Fail 1:251–257. https://doi.org/10.1016/S1388-9842(99)00015-X
Heywood JT, Jermyn R, Shavelle D et al (2017) Impact of practice-based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation 135:1509–1517. https://doi.org/10.1161/CIRCULATIONAHA.116.026184
Temporelli PL, Scapellato F, Eleuteri E et al (2010) Doppler echocardiography in advanced systolic heart failure: a noninvasive alternative to Swan-Ganz Catheter. Circ Heart Fail 3:387–394. https://doi.org/10.1161/CIRCHEARTFAILURE.108.809590
Nagueh SF, Bhatt R, Vivo RP et al (2011) Echocardiographic evaluation of hemodynamics in patients with decompensated systolic heart failure. Circ Cardiovasc Imaging 4:220–227. https://doi.org/10.1161/CIRCIMAGING.111.963496
Yancy CW, Jessup M, Bozkurt B et al (2017) 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of Amer. Circulation 136:e137–e161. https://doi.org/10.1161/CIR.0000000000000509
Balaney B, Medvedofsky D, Mediratta A, et al (2018) HHS Public Access. 31:79–88https://doi.org/10.1016/j.echo.2017.09.002.Invasive
Albakri A (2019) Low-output heart failure: a review of clinical status and meta-analysis of diagnosis and clinical management methods. Clin Med Investig. https://doi.org/10.15761/cmi.1000179
Mele D, Pestelli G, Dini FL et al (2020) Novel echocardiographic approach to hemodynamic phenotypes predicts outcome of patients hospitalized with heart failure. Circ Cardiovasc Imaging. https://doi.org/10.1161/CIRCIMAGING.119.009939
Abbas AE, Khoury Abdulla R, Aggrawal A et al (2017) A novel echocardiographic hemodynamic classification of heart failure based on stroke volume index and left atrial pressure. Echocardiography 34:1417–1425. https://doi.org/10.1111/echo.13642
Kim KH, Jentzer JC, Wiley BM et al (2021) Diamond-Forrester classification using echocardiography haemodynamic assessment in cardiac intensive care unit patients. ESC Heart Fail. https://doi.org/10.1002/ehf2.13527
Simioniuc A, Carluccio E, Ghio S et al (2016) Echo and natriuretic peptide guided therapy improves outcome and reduces worsening renal function in systolic heart failure: an observational study of 1137 outpatients. Int J Cardiol 224:416–423. https://doi.org/10.1016/j.ijcard.2016.09.034
Pugliese NR, Fabiani I, Santini C et al (2019) Value of combined cardiopulmonary and echocardiography stress test to characterize the haemodynamic and metabolic responses of patients with heart failure and mid-range ejection fraction. Eur Heart J Cardiovasc Imaging 20:828–836. https://doi.org/10.1093/ehjci/jez014
Pugliese NR, Fabiani I, Mandoli GE et al (2019) Echo-derived peak cardiac power output-to-left ventricular mass with cardiopulmonary exercise testing predicts outcome in patients with heart failure and depressed systolic function. Eur Heart J Cardiovasc Imaging 20:700–708. https://doi.org/10.1093/ehjci/jey172
Fabiani I, Pugliese NR, Galeotti GG et al (2019) The added value of exercise stress echocardiography in patients with heart failure. Am J Cardiol 123:1470–1477. https://doi.org/10.1016/j.amjcard.2019.02.008
Pugliese NR, De Biase N, Gargani L et al (2020) Predicting the transition to and progression of heart failure with preserved ejection fraction: a weighted risk score using bio-humoural, cardiopulmonary, and echocardiographic stress testing. Eur J Prev Cardiol. https://doi.org/10.1093/eurjpc/zwaa129
Pugliese NR, De Biase N, Conte L et al (2021) Cardiac reserve and exercise capacity: insights from combined cardiopulmonary and exercise echocardiography stress testing. J Am Soc Echocardiogr 34:38–50. https://doi.org/10.1016/j.echo.2020.08.015
Pugliese NR, Paneni F, Mazzola M et al (2021) Impact of epicardial adipose tissue on cardiovascular hemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail. https://doi.org/10.1002/EJHF.2337
Lupón J, Díez-López C, de Antonio M et al (2017) Recovered heart failure with reduced ejection fraction and outcomes: a prospective study. Eur J Heart Fail 19:1615–1623. https://doi.org/10.1002/ejhf.824
van den Berg VJ, Strachinaru M, Akkerhuis KM et al (2019) Repeated echocardiograms do not provide incremental prognostic value to single echocardiographic assessment in minimally symptomatic patients with chronic heart failure: results of the bio-SHiFT study. J Am Soc Echocardiogr 32:1000–1009. https://doi.org/10.1016/j.echo.2019.04.419
Tschöpe C, Kasner M (2014) Can speckle-tracking imaging improve the reliability of echocardiographic parameters for outcome evaluation in clinical trials? Eur Heart J 35:605–607. https://doi.org/10.1093/eurheartj/eht217
Ritzema JL, Richards AM, Crozier IG et al (2011) Serial doppler echocardiography and tissue doppler imaging in the detection of elevated directly measured left atrial pressure in ambulant subjects with chronic heart failure. JACC Cardiovasc Imaging 4:927–934. https://doi.org/10.1016/j.jcmg.2011.07.004
Jones R, Varian F, Alabed S et al (2021) Meta-analysis of echocardiographic quantification of left ventricular filling pressure. ESC Heart Fail 8:566–576. https://doi.org/10.1002/ehf2.13119
Andersen OS, Smiseth OA, Dokainish H et al (2017) Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 69:1937–1948. https://doi.org/10.1016/j.jacc.2017.01.058
Mele D, Andrade A, Bettencourt P et al (2020) From left ventricular ejection fraction to cardiac hemodynamics: role of echocardiography in evaluating patients with heart failure. Heart Fail Rev 25:217–230
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dini, F.L., Ballo, P., Pugliese, N.R. et al. Improved diastolic dysfunction is associated with higher forward flow and better prognosis in chronic heart failure. Int J Cardiovasc Imaging 38, 727–737 (2022). https://doi.org/10.1007/s10554-021-02457-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-021-02457-z