Abstract
Left atrial (LA) volume and function (LA ejection fraction, LAEF) have demonstrated prognostic value in various cardiovascular diseases. We investigated the incremental value of LA volume and LAEF as measured by cardiovascular magnetic resonance imaging (CMR) for prediction of appropriate implantable cardioverter defibrillator (ICD) shock or all-cause mortality, in patients with ICD. We conducted a retrospective, multi-centre observational cohort study of patients who underwent CMR prior to primary or secondary prevention ICD implantation. A single, blinded reader measured maximum LA volume index (maxLAVi), minimum LA volume index (minLAVi), and LAEF. The primary outcome was a composite of independently adjudicated appropriate ICD shock or all-cause death. A total of 392 patients were enrolled. During a median follow-up time of 61 months, 140 (35.7%) experienced an appropriate ICD shock or died. Higher maxLAVi and minLAVi, and lower LAEF were associated with greater risk of appropriate ICD shock or death in univariate analysis. However, in multivariable analysis, LAEF (HR 0.92 per 10% higher, 95% CI 0.81–1.04, p = 0.17) and maxLAVi (HR 1.02 per 10 ml/m2 higher, 95% CI 0.93–1.12, p = 0.72) were not independent predictors of the primary outcome. In conclusion, LA volume and function measured by CMR were univariate but not independent predictors of appropriate ICD shocks or mortality. These findings do not support the routine assessment of LA volume and function to refine risk stratification to guide ICD implant. Larger studies with longer follow-up are required to further delineate the clinical implications of LA size and function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The implantable cardioverter-defibrillator (ICD) has been established as an effective therapy for both primary and secondary prevention of sudden cardiac death (SCD) [1]. SCD accounts for up to 50% of cardiovascular mortality, and patients with left ventricular (LV) systolic dysfunction are recognized to be at significantly higher risk [2,3,4]. Consequently, guidelines recommend using LV ejection fraction (LVEF) to determine prophylactic ICD eligibility [1].
Although LVEF, late-gadolinium enhancement (LGE) and clinical variables such as New York Heart Association (NYHA) class have demonstrated utility as risk stratifiers, they are imperfect for selecting patients who would most likely benefit from a primary prevention ICD. Prior studies demonstrate that a quarter of patients with ICD experience appropriate ICD shocks within 5 years [5, 6]. In another contemporary real-world registry, only 1.1% and 3.8% of patients received appropriate shocks at 1 year in the setting of primary and secondary prevention, respectively [7]. The DANISH study conducted in patients with non-ischemic dilated cardiomyopathy (NICM) showed that prophylactic ICD implantation was not associated with mortality reduction [8]. Collectively, these data highlight the need for additional prognostic markers that can improve appropriate patient selection for ICD [2, 9].
Novel imaging parameters have been investigated for prediction of arrhythmic events and SCD, of which LV scar characteristics have been most extensively investigated [10,11,12]. Left atrial (LA) size and function have emerged as predictors of atrial arrhythmias and adverse heart failure and cardiovascular outcomes [13, 14]. Indeed, LA enlargement has been recognized as an indicator of the severity and chronicity of LV diastolic dysfunction and elevated filling pressures [15,16,17]. Recently, LA function has been identified as a predictor of SCD or appropriate ICD shock [18, 19]. However, there are insufficient data to confirm the incremental value of LA function (beyond LVEF and LGE) as a marker of ventricular arrhythmic events following ICD implantation. Accordingly, we sought to determine whether LA volume and/or function independently predicts appropriate ICD shock or all-cause mortality.
Methods
Study design
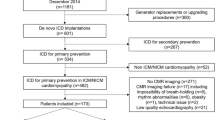
We conducted a multi-centred, retrospective, observational cohort study of patients who required an ICD for either primary or secondary prevention at two tertiary care hospitals (St. Michael’s Hospital and Sunnybrook Health Sciences Center in Toronto, Canada) between January 2007 and May 2017. The study was approved by the Research Ethics Boards at all study sites. Informed consent was waived for this retrospective study.
We included patients who underwent ICD implantation for either ischemic cardiomyopathy or non-ischemic cardiomyopathy, who were at least 18 years old and had a cardiovascular magnetic resonance imaging (CMR) performed pre-implant. Patients were categorized as ischemic cardiomyopathy if they had a history of myocardial infarction (MI), percutaneous coronary intervention (PCI), bypass surgery, or known CAD. Patients with arrhythmogenic right ventricular dysplasia (ARVD) and channelopathies (e.g. long QT syndrome) were excluded. Patient demographics, comorbidities, medical therapy and appropriate ICD shock data were collected by individual chart review. ICD programming was at the discretion of the treating physician as in standard clinical practice. All CMR analysis was blinded to patient clinical characteristics and outcomes.
Outcome
For our primary outcome, we chose to focus on harder endpoints, namely composite of appropriate ICD shock or all-cause mortality, as opposed to anti-tachycardia pacing which is highly dependent on device programming. We chose this definition to allow us to evaluate the impact of LA volume and function on more definitive outcomes. Patients were followed prospectively both in the device clinic at 6-month intervals and/or followed-up more urgently based on home monitoring events. All ICD shocks (appropriate versus inappropriate) were reviewed by trained device technicians and by an attending electrophysiologist blinded to LA measurements. Deaths were determined using electronic chart review, hospital and autopsy records, or confirmed by the primary care provider. Patients who did not develop the corresponding endpoint by the end of observation period were censored at the last clinic follow-up.
CMR image processing
Baseline CMR was performed following recruitment, prior to ICD insertion. All CMR examinations were performed with a 1.5 T scanner (Intera, Philips Medical Systems, Netherlands or TwinSpeed Excite, GE Healthcare, Milwaukee, Wisconsin) using a cardiac coil and retrospective electrocardiographic gating. The Philips 1.5 T scanner used a 5-channel (SENSE) cardiac coil and the GE scanner used a standard 8-channel cardiac coil.
Standard protocols using validated, commercially available sequences were used. Images were obtained with breath-hold at end-expiration. Standard long-axis two-, four-, and three-chamber views were obtained, as well as sequential short-axis covering the LV. Steady-state free precession sequence (SSFP) was used to acquire cine images in long-axis planes followed by sequential short-axis cine loops with the following typical parameters: repetition time 4 ms, time to echo 2 ms, slice thickness 8 mm, field of view 320–330 × 320-330 mm (tailored to achieve optimal spatial resolution and image acquisition time), matrix size 256 × 196, temporal resolution of < 40 ms (depending on the heart rate) and flip angle 50 degrees. LGE images were obtained for myocardial scar assessment, about 15 min following the administration of gadolinium using a segmented inversion-recovery gradient echo sequence. Prior to image processing, all CMR studies were de-identified and assigned a unique identification code. CMR studies were analyzed with commercially available cvi42 software (Circle Cardiovascular, Calgary, Canada).
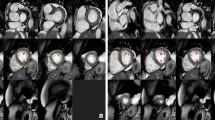
The biplane area-length method was used to estimate LA volume (LAV) [20]. Manual measurements for LAV were made at its maximal size (maxLAV), at end-ventricular systole in the frame prior to mitral valve opening using the following formula: LAV = 8(A2)(A4)/3πL, where A2 and A4 represent the LA areas in the two- and four-chamber views, respectively, and L is the average of the two LA lengths in each respective view measured from the midpoint of the mitral annulus to the posterior aspect of the LA (Fig. 1). The minimal LAV (minLAV) was measured in the same manner except at end-ventricular diastole in the frame prior to mitral valve closure. When tracing the LA endocardial borders, the pulmonary veins and LA appendage were excluded. The LA ejection fraction (LAEF) was estimated using the biplane area-length method: LAEF = (maxLAV–minLAV)/maxLAVx100%. The LA volumes maxLAV and minLAV were indexed to body surface area (maxLAVi and minLAVi, respectively).
LV end-diastolic volumes (LVEDV) and LV end-systolic volumes (LVESV) were measured using the short-axis cine images by manually tracing endocardial contours during ventricular end-diastole and end-systole, using the blood volume method that included papillary muscles and trabeculations. LVEF was calculated as (LVEDV-LVESV)/LVEDVx100%. LV mass (LVM) was calculated using the area between the endocardial and epicardial borders multiplied by the slice thickness and interslice distance, using contiguous short-axis slices at end-diastole [21]. LVEDV, LVESV, and LVM were also indexed to body surface area. A single reader measured LA parameters in random order, whereas LV measurements and LGE assessment were performed independently by 2 other CMR readers.
Statistical analysis
Continuous data are expressed as mean with standard deviation or median with interquartile range (IQR), as appropriate. Non-parametric Mann–Whitney U test was used to compare continuous data between groups. Chi-square or Fisher's exact test was used to compare categorical variables between groups. The relationships between CMR parameters were examined using non-parametric Spearman’s correlation test.
Kaplan–Meier method was used to display the time to primary outcome of appropriate ICD shocks or all-cause death, according to quartiles of LA volumes and LAEF, and compared by log-rank test for trend. Cox proportional hazards model was used to examine the association between CMR LA parameters and outcome, adjusting for age, LVEF, ICD indication (primary vs. secondary), and presence or absence (binary variable) of LGE as surrogate of LV scar. Separate models were performed to examine the independent impact of maxLAVi and LAEF on composite outcome of ICD shock or death, or death alone (four models). We tested for interactions between LA parameters (maxLAVi and LAEF) and primary vs. secondary indication for ICD, as well as presence vs absence of CAD. Restricted cubic splines were used to model potentially non-linear relationship between covariates and outcome. To examine for potential collinearity, we calculated variance inflation factors which were all < 2. Lastly, sensitivity analysis was conducted for patients without a history of atrial fibrillation.
To determine intra-observer and inter-observer reproducibility, a random sample of 25 and 20 CMRs were measured by the same reader and an independent reader, respectively. Statistical significance was defined as a two-sided p value < 0.05. All data were analyzed using SPSS version 22 (IBM Corp., Armonk, NY) and STATA version 15.1 (Stata Corp, College Station, TX).
Results
A total of 392 patients with ICD and baseline CMR were included in this study. Table 1 outlines the baseline characteristics. The mean age of this cohort was 60 years old and 79% were men; 62% of patients received an ICD for primary prevention, 57% had CAD, and 32% had history of atrial fibrillation. The mean maxLAVi and minLAVi was 45.8 and 29.7 mL/m2, respectively. The mean LAEF was 38.3% and 70% of patients had evidence of LGE. The intra-observer reproducibility was excellent, with intraclass correlation coefficients for absolute agreement of 0.99 (95% CI 0.99–1.00) for maxLAV and 0.97 (95% CI 0.92–0.99) for LAEF. The inter-observer reproducibility was also very good, with intraclass correlation coefficients for absolute agreement of 0.89 (95% CI 0.68–0.96) for maxLAV and 0.96 (95% CI 0.90–0.98) for LAEF.
Patients with history of atrial fibrillation had significantly higher maxLAVi (53.1 ± 22.2 vs. 42.4 ± 16.7 mL/m2, p < 0.001), minLAVi (38.5 ± 22.6 vs. 25.6 ± 15.7 mL/m2, p < 0.001), and lower LAEF (31.6 ± 16.3% vs. 43.1 ± 15.3%, p < 0.001) compared to those without atrial fibrillation.
The median time from pre-implantation CMR to ICD insertion was 28 days. During a median follow-up time of 61 months (interquartile range 38 to 102 months), 91 patients (23.3%) experienced an appropriate ICD shock, 72 patients died (18.4%), and 140 patients (35.7%) reached the primary composite endpoint of appropriate ICD shock or death.
The correlations between LA parameters and LV parameters on CMR are summarized in Table 2. MaxLAVi and minLAVi were positively correlated with LVEDVi and LVESVi, as well as LVMi. LA volumes were negatively correlated with LVEF, whereby higher minLAVi and maxLAVi were associated with worse LVEF. LAEF was negatively correlated with LVEDVi, LVESVi, and LVMi, whereby lower LAEF was associated with higher LVEDVi, LVESVi, and LVMi. LAEF was positively correlated with LVEF.
In univariate analysis, higher maxLAVi (Fig. 2a), higher minLAVi (Fig. 2b), and lower LAEF (Fig. 2c) were all associated with appropriate ICD shock or all-cause mortality. Although LA parameters were correlated with LVEF, these correlations were not high enough to preclude entry into models including LVEF due to collinearity (all variance inflation factors < 2.0).
Multivariable Cox regression showed neither LAEF (HR 0.92 per 10% higher, 95% confidence interval [CI] 0.81–1.04, p = 0.17) nor maxLAVi (HR 1.02 per 10 mL/m2 higher, 95% CI 0.93–1.12, p = 0.72) to be independent predictors of composite outcome (Table 3). Similarly, LAEF and maxLAVi were not independent predictors of all-cause mortality alone (LAEF 0.98 per 10% higher, 95% CI 0.82–1.16, p = 0.79; maxLAVi HR 1.05 per 10 mL/m2 higher, 95% CI 0.93–1.20, p = 0.44) (Table 3). LVEF was an independent predictor of outcomes while presence of scar was not (Table 3). There was no significant interaction between maxLAVi and LAEF and primary vs. secondary ICD indication, and known vs. no known CAD. Ancillary analysis showed that similar to LAEF and maxLAVi, minLAVi was also not an independent predictor of the composite of death/appropriate ICD shock (HR 1.03 per 10 mL/m2 higher, 95% CI 0.97–1.13, p = 0.61) or all-cause mortality (HR 1.03 per 10 mL/m2 higher, 95% CI 0.90–1.17, p = 0.69).
Lastly, multivariable Cox regression with the use of restricted cubic splines did not show clear departure from linearity, indicating that maxLAVi and LAEF can be modelled linearly. The results remain unchanged in the sensitivity analysis excluding patients with history of atrial fibrillation (data not shown).
Discussion
Among patients who received an ICD for primary or secondary prevention, larger LA volumes were associated with reduced LAEF and LVEF, and there was a positive correlation between LAEF and LVEF. Although higher LA volumes and lower LAEF were associated with appropriate ICD shock or all-cause mortality in univariate analyses, these associations were no longer significant after adjusting for age, presence of LGE and LVEF. Taken together, our results suggest that LA volume and function do not provide incremental predictive value for appropriate ICD shocks and mortality in patients who underwent ICD implantation.
As LA function is closely intertwined with LV volume and filling pressures [13], there is emerging evidence for the predictive value of LA size and function for risk stratification of cardiovascular diseases [22,23,24]. Herein, we observed correlations between LAV and function, and LV volumes, mass, and function. These findings are not surprising given the hemodynamics between atria and ventricles are interconnected. When LV compliance is reduced, filling pressure increases in the LV and thereby, increasing LA pressure leading to structural remodelling [25]. Accordingly, adverse remodelling of the LA leading to LA dysfunction may be an important marker of more severe LV dysfunction and adverse outcomes. Indeed, in both HF with reduced and preserved ejection fraction, LA filling pressures are increased and associated with ventricular arrhythmias [26, 27].
Currently, there are limited data on the role of LA imaging parameters to predict ICD treatment response. A recent study by Rijnierse et al. evaluated the value of LAV and LAEF by CMR in 229 patients (166 had LGE assessment) who received an ICD for primary prevention of SCD [19]. They found that although minimum LAV was a significant predictor of the primary outcome of first appropriate device therapy in univariate analysis, LAEF and LV scar were independent and incremental predictors of the arrhythmic outcome. Similarly, a more recent study by Lydell et al. found that LAEF was a strong and independent predictor of ventricular arrhythmia beyond LVEF in patients with reduced LVEF who underwent CMR imaging prior to primary prevention ICD implantation [18].
In this study, CMR-derived LAV and LAEF were univariate but not independent predictors of appropriate ICD shocks or mortality. These contrasting findings to studies outlined above may be related to differences in patient characteristics or adjudicated outcomes. Here, we included patients who received an ICD for both primary and secondary prevention, as compared to inclusion of only patients who required primary prevention ICD implantation. The outcome definition also differs. The outcome of interest in Rijnierse et al. was appropriate ICD shock, and the outcome in Lydell et al. was a composite of SCD and appropriate ICD shock [18, 19]. Additionally, of note, Rijnierse et al. incorporated anti-tachycardia pacing as appropriate therapy, a less rigorous endpoint than an ICD shock. Conversely, in our study, we evaluated all-cause mortality which is a more robust outcome measure, because the cause of death is often difficult to discern. Our outcome definition is congruent with that of clinical trials of ICD implantation using all-cause mortality as the primary outcome. The present study included a greater number of events (140 ICD shocks or deaths) over a longer follow-up period compared to Rijnierse et al.(40 anti-tachycardia pacing therapies and 22 ICD shocks) and Lydell et al.(35 events)[18, 19]. Furthermore, LAEF was found to be an independent predictor of adverse outcome in multivariable analysis that only adjusted for diuretic use [18]. In contrast, our findings do not support the routine use of CMR quantified LA volume and LAEF as independent predictors of appropriate ICD shock or all-cause mortality for risk stratification. Future larger studies with longer follow-up are required to delineate the prognostic utility of LAEF for adverse outcomes in this setting.
To rigorously assess the incremental predictive value of LA parameters, we adjusted for LVEF and LGE in our multivariable analysis. While reduced LVEF was a significant predictor of appropriate ICD shock or all-cause mortality in this study, it was not included in the previous studies [18, 19]. Although statistical significance was reached for LVEF, it is likely not a strong predictor of arrhythmia outcomes. Patients selected for ICD implantation were primarily based on reduction in LVEF; hence, differences in LVEF below 30–35% may not be as powerful predictors of appropriate ICD shocks. Studies have also suggested that LVEF alone is likely imperfect for selection of patients most likely to benefit from primary prevention ICDs. While ICDs have been shown to reduce mortality among patients with LVEF dysfunction due to MI, the evidence is less clear for those with NICM [28]. Given the cost and potential complications of ICD, better selection of patients at high risk of SCD is required.
There are several strengths of our study. This cohort included patients with few exclusion criteria and CMR were performed using different vendors in 2 centres, increasing the generalizability of our results. CMR measurements were performed by independent, blinded readers, with good intra-observer reproducibility. Compared to echocardiography, chamber quantification with CMR is more precise, and considered the gold standard [29, 30]. Furthermore, CMR has better endocardial border definition and may yield more accurate LA measurements, compared with echocardiogram [31]. Although there are various reported methods for estimating LAV including biplane disk summation, and prolate ellipse methods [32, 33], we chose to use the biplane area-length method as most images did not contain dedicated atrial short axis slices. The prolate ellipse method generally underestimates LAV, while the biplane area-length and biplane disk summation techniques correlate better with true LAV [34]. However, a disk summation technique is more cumbersome and clinically impractical, and many outcome studies use the biplane area-length technique, which allows for more direct comparisons across studies, justifying our use of biplane area-length technique here.
Despite these strengths, our study has several limitations. Although we did not find any significant association between LA size and LAEF and outcomes, this may be attributable to inadequate sample size and power. Nevertheless, our study included a greater number of events than previous studies that reported a positive association, albeit using different endpoints. Despite the rationale that risk stratification for SCD may be more beneficial in individuals who have not yet had a cardiac arrest, we chose to include patients who received an ICD for both primary and secondary prevention a priori, which were controlled for in the multivariable model. This observational study was conducted at two tertiary care centres and included only patients who received an ICD as per clinical indication; as such, the prognostic value of LA parameters among patients who did not qualify for an ICD is beyond the scope of this study. However, since all patients in this study received an ICD, the outcome of a serious arrhythmia requiring shock was readily apparent for all patients enrolled. Our data lacked information on NYHA status and HF is an important confounder. We did not evaluate the effect of other LA parameters such as phasic LA function on outcomes.
Conclusions
Among patients with ICD for primary or secondary prophylaxis, LA size and function were not independent predictors of appropriate shocks or all-cause mortality after adjusting for LVEF and other clinical features. LA size and function correlated with left ventricular size and function. Overall, our findings do not support the routine use of LA parameters on CMR to refine risk stratification for ICD patients. Larger studies with longer follow-up may further delineate the prognostic value of LA size and function in a broad spectrum of patients being considered for ICD implant.
Data availability
Data available upon request.
References
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB et al (2018) 2017 AHA/ACC/HRS 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart Rhythm society. J Am Coll Cardiol 72(14):1677–1749. https://doi.org/10.1016/j.jacc.2017.10.053
Goldberger JJ, Subacius H, Patel T, Cunnane R, Kadish AH (2014) Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol 63(18):1879–1889. https://doi.org/10.1016/j.jacc.2013.12.021
Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R et al (2005) Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 352(3):225–237. https://doi.org/10.1056/NEJMoa043399
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS et al (2002) Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346(12):877–883. https://doi.org/10.1056/NEJMoa013474
Ezekowitz JA, Rowe BH, Dryden DM, Hooton N, Vandermeer B, Spooner C et al (2007) Systematic review: implantable cardioverter defibrillators for adults with left ventricular systolic dysfunction. Ann Intern Med 147(4):251–262. https://doi.org/10.7326/0003-4819-147-4-200708210-00007
Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT et al (2009) Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation 120(10):835–842. https://doi.org/10.1161/CIRCULATIONAHA.108.816884
Sabbag A, Suleiman M, Laish-Farkash A, Samania N, Kazatsker M, Goldenberg I et al (2015) Contemporary rates of appropriate shock therapy in patients who receive implantable device therapy in a real-world setting: from the Israeli ICD Registry. Heart Rhythm 12(12):2426–2433. https://doi.org/10.1016/j.hrthm.2015.08.020
Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E et al (2016) DefibrillatorDefibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 375(13):1221–1230. https://doi.org/10.1056/NEJMoa1608029
Santangeli P, Dello Russo A, Casella M, Pelargonio G, Di Biase L, Natale A (2011) Left ventricular ejection fraction for the risk stratification of sudden cardiac death: friend or foe? Intern Med J 41(1a):55–60. https://doi.org/10.1111/j.1445-5994.2010.02371.x
Almehmadi F, Joncas SX, Nevis I, Zahrani M, Bokhari M, Stirrat J et al (2014) Prevalence of myocardial fibrosis patterns in patients with systolic dysfunction: prognostic significance for the prediction of sudden cardiac arrest or appropriate implantable cardiac defibrillator therapy. Circ Cardiovasc Imaging 7(4):593–600. https://doi.org/10.1161/CIRCIMAGING.113.001768
Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S et al (2013) Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 309(9):896–908. https://doi.org/10.1001/jama.2013.1363
Haghbayan H, Lougheed N, Deva DP, Chan KKW, Lima JAC, Yan AT (2019) Peri-Infarct quantification by cardiac magnetic resonance to predict outcomes in ischemic cardiomyopathy: prognostic systematic review and meta-analysis. Circ Cardiovasc Imaging 12(11):e009156. https://doi.org/10.1161/CIRCIMAGING.119.009156
Hoit BD (2014) Left atrial size and function: role in prognosis. J Am Coll Cardiol 63(6):493–505. https://doi.org/10.1016/j.jacc.2013.10.055
Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB (2002) Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 90(12):1284–1289. https://doi.org/10.1016/s0002-9149(02)02864-3
Appleton CP, Galloway JM, Gonzalez MS, Gaballa M, Basnight MA. Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol. 1993;22(7):1972–82. https://doi.org/10.1016/0735-1097(93)90787-2.
Geske JB, Sorajja P, Nishimura RA, Ommen SR (2009) The relationship of left atrial volume and left atrial pressure in patients with hypertrophic cardiomyopathy: an echocardiographic and cardiac catheterization study. J Am Soc Echocardiogr 22(8):961–966. https://doi.org/10.1016/j.echo.2009.05.003
Guron CW, Hartford M, Rosengren A, Thelle D, Wallentin I, Caidahl K (2005) Usefulness of atrial size inequality as an indicator of abnormal left ventricular filling. Am J Cardiol 95(12):1448–1452. https://doi.org/10.1016/j.amjcard.2005.02.011
Lydell CP, Mikami Y, Homer K, Peng M, Cornhill A, Rajagopalan A et al (2019) Left atrial function using cardiovascular magnetic resonance imaging independently predicts life-threatening arrhythmias in patients referred to receive a primary prevention implantable cardioverter defibrillator. Can J Cardiol 35(9):1149–1157. https://doi.org/10.1016/j.cjca.2019.04.015
Rijnierse MT, Kamali Sadeghian M, Schuurmans Stekhoven S, Biesbroek PS, van der Lingen AC, van de Ven PM et al (2017) Usefulness of left atrial emptying fraction to predict ventricular arrhythmias in patients with implantable cardioverter defibrillators. Am J Cardiol 120(2):243–250. https://doi.org/10.1016/j.amjcard.2017.04.015
Ren JF, Kotler MN, DePace NL, Mintz GS, Kimbiris D, Kalman P et al (1983) Two-dimensional echocardiographic determination of left atrial emptying volume: a noninvasive index in quantifying the degree of nonrheumatic mitral regurgitation. J Am Coll Cardiol 2(4):729–736. https://doi.org/10.1016/s0735-1097(83)80313-1
Maceira AM, Prasad SK, Khan M, Pennell DJ (2006) Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 8(3):417–426
Fatema K, Barnes ME, Bailey KR, Abhayaratna WP, Cha S, Seward JB, et al. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr. 2009;10(2):282–6. https://doi.org/10.1093/ejechocard/jen235.
Habibi M, Samiei S, Ambale Venkatesh B, Opdahl A, Helle-Valle TM, Zareian M, et al. Cardiac magnetic resonance-measured left atrial volume and function and incident atrial fibrillation: results from MESA (Multi-Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging. 2016;9(8). https://doi.org/10.1161/CIRCIMAGING.115.004299.
Pellicori P, Zhang J, Lukaschuk E, Joseph AC, Bourantas CV, Loh H et al (2015) Left atrial function measured by cardiac magnetic resonance imaging in patients with heart failure: clinical associations and prognostic value. Eur Heart J 36(12):733–742. https://doi.org/10.1093/eurheartj/ehu405
Huang JL, Tai CT, Lin YJ, Ting CT, Chen YT, Chang MS et al (2006) The mechanisms of an increased dominant frequency in the left atrial posterior wall during atrial fibrillation in acute atrial dilatation. J Cardiovasc Electrophysiol 17(2):178–188. https://doi.org/10.1111/j.1540-8167.2005.00297.x
Kayatas M, Ozdemir FN, Muderrisoglu H, Korkmaz ME (1999) Diastolic dysfunction increases the frequency of ventricular arrhythmia in hemodialysis patients. Nephron 82(2):185–187. https://doi.org/10.1159/000045398
Mordi I, Jhund PS, Gardner RS, Payne J, Carrick D, Berry C et al (2014) LGE and NT-proBNP identify low risk of death or arrhythmic events in patients with primary prevention ICDs. JACC Cardiovasc Imaging 7(6):561–569. https://doi.org/10.1016/j.jcmg.2013.12.014
Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793–867. https://doi.org/10.1093/eurheartj/ehv316.
Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ (2000) Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2(4):271–278. https://doi.org/10.3109/10976640009148691
Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU et al (2002) Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 90(1):29–34. https://doi.org/10.1016/s0002-9149(02)02381-0
Rodevan O, Bjornerheim R, Ljosland M, Maehle J, Smith HJ, Ihlen H (1999) Left atrial volumes assessed by three- and two-dimensional echocardiography compared to MRI estimates. Int J Card Imaging 15(5):397–410
Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S (2005) Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson 7(5):775–782. https://doi.org/10.1080/10976640500295516
Sievers B, Kirchberg S, Addo M, Bakan A, Brandts B, Trappe HJ (2004) Assessment of left atrial volumes in sinus rhythm and atrial fibrillation using the biplane area-length method and cardiovascular magnetic resonance imaging with TrueFISP. J Cardiovasc Magn Reson 6(4):855–863. https://doi.org/10.1081/jcmr-200036170
Jiamsripong P, Honda T, Reuss CS, Hurst RT, Chaliki HP, Grill DE et al (2008) Three methods for evaluation of left atrial volume. Eur J Echocardiogr 9(3):351–355. https://doi.org/10.1016/j.euje.2007.05.004
Funding
No external funding has been obtained for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The study was approved by the Research Ethics Boards at study sites St. Michael’s Hospital and Sunnybrook Health Sciences Center in Toronto, Ontario, Canada.
Consent to participate
Informed consent was waived for this retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gong, I.Y., Yazdan-Ashoori, P., Jimenez-Juan, L. et al. Left atrial volume and function measured by cardiac magnetic resonance imaging as predictors of shocks and mortality in patients with implantable cardioverter-defibrillators. Int J Cardiovasc Imaging 37, 2259–2267 (2021). https://doi.org/10.1007/s10554-021-02196-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-021-02196-1