Abstract
The sequential or concurrent use of two different types of agents such as anthracyclines and trastuzumab may increase myocardial injury and cancer therapeutics-related cardiac dysfunction (CTRCD), which is often the result of the combined detrimental effect of the two therapies for breast cancer patients. However, the association between clinical risk factors and left ventricular (LV) function in such patients is currently unclear. We studied 86 breast cancer patients with preserved LV ejection fraction (LVEF) and treated with anthracyclines, trastuzumab, or both. Echocardiography was performed before and 16 days after chemotherapy. In accordance with the current position paper, clinical risk factors for CTRCD were defined as: cumulative dose of doxorubicin > 240 mg/m2, age > 65-year-old, body mass index > 30 kg/m2, previous radiation therapy, B-type natriuretic peptide > 100 pg/mL, previous history of cardiovascular disease, atrial fibrillation, hypertension, diabetes, and smoking. The relative decrease in LVEF after chemotherapy for patients with more than four risk factors was significantly greater than that for patients without (− 9.3 ± 10.8% vs. − 2.2 ± 10.2%; p = 0.02). However, this finding did not apply to patients with more than one, two or three risk factors. Patients with more than four risk factors also tended to show a higher prevalence of CTRCD than those without (14.3% vs. 2.8%; p = 0.12). Moreover, the relative decrease in LVEF became greater as the number of risk factors increased. This study found multiple risk factors were associated with LV dysfunction following chemotherapy. Our findings can thus be expected to have clinical implications for better management of patients with breast cancer referred for chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advances in treatment have led to improved survival of cancer patients, but have also increased morbidity and mortality due to treatment side effects [1, 2]. Cardiovascular diseases are some of the most frequent of these side effects, and there is a growing concern that they may lead to premature morbidity and death among cancer survivors [3]. This may be the result of cardiotoxicity, which involves direct effects of the cancer treatment on left ventricular (LV) function and structure or may be due to accelerated development of cardiovascular diseases. Notably, the mortality rate for patients with cancer therapeutics-related cardiac dysfunction (CTRCD) is reportedly as high as 60% by 2 years after treatment [4]. However, if CTRCD is detected early and treated with heart failure (HF) medications, patients frequently have a good functional recovery [5]. Thus, early detection of CTRCD is essential for avoiding or delaying progression to HF in patients with a history of using cardiotoxins, but its assessment can be challenging.
For patients with breast cancer, the sequential or concurrent use of two different types of agents such as anthracyclines and trastuzumab may increase LV myocardial injury, while CTRCD is often the result of the combined detrimental effect of the two therapies. Furthermore, breast cancer was found to be the cumulative primary cause of death during the first 5 to 10 years following breast cancer diagnosis, whereas cardiovascular diseases became the cumulative primary cause of death during longer follow-ups [6]. For risk stratification to detect the development of CTRCD, the current position paper from the European Society of Cardiology (ESC) lists several factors associated with risk of cardiotoxicity following treatment with chemotherapy [2]. However, details of the association between these clinical risk factors for CTRCD and LV function in patients with breast cancer remain unclear. Accordingly, the aim of this study was to investigate the impact of baseline risk factors on LV function in patients with preserved LV ejection fraction (LVEF) who have undergone anthracycline or trastuzumab chemotherapy for breast cancer.
Materials and methods
Study population
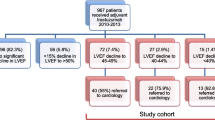
We retrospectively enrolled 206 consecutive patients with breast cancer treated at Kobe University Hospital between June 2008 and May 2019. Excluded were patients with: (1) no administration of anthracyclines or trastuzumab; (2) no echocardiographic examination before or after anthracycline and trastuzumab chemotherapy; (3) LV systolic dysfunction before chemotherapy, defined as a LVEF < 50%. After exclusion of the above patients, the patient study group consisted of 86 patients with breast cancer. This study was approved by the Local Ethics Committee of our institution in conformity with the Declaration of Helsinki (No. B190263).
Echocardiography
Echocardiographic studies were performed before and median 16 (1–61) days after completion of chemotherapy by using a commercially available echocardiography system (Aplio Artida, Aplio 400 and Xario; Canon Medical Systems, Tochigi, Japan, Vivid 7 and E9; GE-Vingmed, Horten, Norway, and iE33; Philips Medical Systems, Andover, MA). Digital routine grayscale two-dimensional cine loops from three consecutive heart beats were obtained at end-expiratory apnea from standard parasternal and apical views. Sector width was optimized to allow for complete myocardial visualization while maximizing the frame rate. Standard echocardiographic measurements were obtained in accordance with the current guidelines of the American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging (EACVI) [7].
Speckle-tracking strain analysis was performed for each patient with the aid of a single dedicated software to evaluate LV longitudinal function, which was assessed in terms of global longitudinal strain (GLS, AutoSTRAIN, TOMTEC-ARENA; TOMTEC Imaging Systems GmbH, Unterschleissheim, Germany). Briefly, apical 4-chamber, 2-chamber and long-axis views with the Digital Imaging and Communications in Medicine (DICOM) formatted file images were uploaded onto a personal computer for subsequent off-line GLS analysis. Longitudinal speckle-tracking strain was calculated by means of an automated contouring detection algorithm, and manual adjustments of regions of interest were performed where necessary. GLS was then determined as the averaged peak longitudinal strain of 18 LV segments, and was expressed as an absolute value in line with current guidelines [7].
Definition of CTRCD
In accordance with the current definition of CTRCD by ASE and the EACVI consensus statement, CTRCD was defined as a decline in LVEF of > 10% to an absolute value of < 53% after the termination of chemotherapy [8].
Definition of risk factors for CTRCD
The baseline risk factors for CTRCD were categorized, based on the current ESC position paper, as (1) a cumulative total doxorubicin dose of > 240 mg/m2, (2) age > 65-year-old, (3) body mass index > 30 kg/m2, (4) a previous history of radiation therapy to chest or mediastinum, (5) B-type natriuretic peptide > 100 pg/mL, (6) a previous history of cardiovascular disease, (7) atrial fibrillation, (8) hypertension, (9) diabetes mellitus, (10) current or ex-smoker [2].
Statistical analysis
Continuous variables were expressed as mean values and standard deviation for normally distributed data, and as the median and interquartile range for non-normally distributed data. Categorical variables were expressed as frequencies and percentages. The parameters of subgroups were compared by means of the Student t test, Mann–Whitney U test, or paired samples t test as appropriate. Proportional differences were compared by means of Fisher’s exact test, χ2 test, or Mcnemar test as appropriate. Sequential logistic models for predicting CTRCD were constructed to determine any incremental benefits of using more than four risk factors compared with using only conventional baseline echocardiographic variables consisting of LVEF, mitral inflow E and mitral e′ annular velocities ratio (E/e′), and mitral inflow E and A velocities ratio (E/A). A statistically significant increase in the global log-likelihood χ2 of the model was defined as an increment in predictive value. The initial univariate logistic regression analysis to identify univariate determinants of developing CTRCD was followed by a multivariate logistic regression model using stepwise selection, with the p levels for entry from the model set at < 0.10. Optimal cutoff value for association of a cumulative total doxorubicin dose with LV dysfunction after chemotherapy were determined with receiver–operator characteristics curve analysis. p value < 0.05 was considered statistically significant for all tests. All the analyses were performed with commercially available software (MedCalc software version 19.1; MedCalc Software, Mariakerke, Belgium).
Results
Baseline characteristics
The baseline clinical and echocardiographic characteristics of the 86 patients with breast cancer are summarized in Table 1. Their mean age was 59 ± 13 years, 85 (99%) were female, and LVEF was 66.5 ± 4.7%.
Comparison of changes in LVEF of patients with and without risk factors
Figure 1 shows changes in LVEF of patients with and without risk factors. The relative decrease in LVEF was became greater as the number of risk factors increased, and the relative decrease in LVEF after chemotherapy of patients with more than four risk factors was significantly greater than that in patients without (− 9.3 ± 10.8% vs. − 2.2 ± 10.2%; p = 0.02). However, this relationship was not observed between patients with more than one (− 3.0 ± 10.5% vs. − 3.2 ± 8.3%; p = 0.97), two (− 3.3 ± 11.2% vs. − 3.4 ± 9.3%; p = 0.96) or three risk factors (− 5.3 ± 11.3% vs. − 1.9 ± 9.6%; p = 0.14) and those without. We did not examine the difference between patients with more than five risk factors and those without because of the very small number (only three) of patients with more than five risk factors. Moreover, patients with more than four risk factors tended to show a higher prevalence of CTRCD compared to those without (14.3% vs. 2.8%; p = 0.12). The comparison of echocardiographic parameters between baseline and after completion of chemotherapy is shown in Table 2. After completion of chemotherapy, LVEF significantly decreased from 66.5 ± 4.7% to 64.1 ± 6.1% (p = 0.002) and LV end-systolic volume significantly increased from 23.5 mL (19.0–27.7) to 24.0 mL (20.0–27.6) (p = 0.02). In addition, the comparison of clinical and echocardiographic characteristics of patients with and without CTRCD are summarized in Table 3. Though small number of patients with CTRCD, patients with CTRCD were more likely to be smoker (p = 0.003), and tended to be higher total cumulative doxorubicin dose (p = 0.08) and higher LV mass index (p = 0.07) compared to those without CTRCD.
Bar graphs showing comparisons of the relative decrease in LVEF after chemotherapy for patients with and without risk factors. The relative decrease for patients with more than four risk factors was significantly higher than that for patients without, but this relationship was not observed between patients with more than one, two or three risk factors and those without
The incremental benefits of using sequential logistic models for the prediction of CTRCD after chemotherapy, show that a model based on baseline conventional echocardiographic variables including LVEF, E/e′, and E/A was significantly improved by the addition of the variable of having more than four risk factors. The addition of baseline GLS tended to result in similar improvement
Associated factors for CTRCD
Table 4 shows the results of the univariate and multiple regression analysis of associated factors for developing CTRCD. LV mass index and more than four risk factors were entered to multiple regression analysis from univariate regression analysis, but none of them were independent parameters. The incremental benefits of using sequential logistic models for the prediction of CTRCD after chemotherapy are shown in Fig. 2. LVEF, E/A and E/e′ were chosen as baseline sequential models because of common global LV function. A model based on baseline conventional echocardiographic variables including LVEF, E/e′, and E/A (χ2 = 0.8) was significantly improved by the addition of the variable of having more than four risk factors (χ2 = 5.9; p = 0.02). Similarly, the addition of baseline GLS tended to result in improvement (χ2 = 2.7; p = 0.17).
Comparison of echocardiographic parameters for patients with more than any of four risk factors and those without
Echocardiographic parameters between patients with more than any of four risk factors and those without are shown in Table 5. Patients with more than four risk factors were more likely to have LV hypertrophy (interventricular septal thickness end-diastole: 10.3 ± 2.1 mm vs. 9.0 ± 1.9 mm; p = 0.04, posterior wall thickness end-diastole: 10.4 ± 1.7 mm vs. 9.3 ± 1.7 mm; p = 0.02, LV mass index: 109.3 ± 29.0 g/m2 vs. 83.2 ± 21.0 g/m2; p < 0.001), and larger left atrial volume index (36.3 ± 11.4 mL/m2 vs. 29.6 ± 10.1 mL/m2; p = 0.03). Furthermore, GLS tended to be lower (18.4 ± 2.8% vs. 20.0 ± 2.6%; p = 0.06) and E/e′ tended to be higher (10.4 [8.9–13.0] vs. 9.0 [7.4–10.9]; p = 0.06) for patients with more than four risk factors compared to those without.
Optimal cut off value of a cumulative total doxorubicin dose for predicting LV dysfunction for patients with more than any of four risk factors and those without
LV dysfunction after completion of chemotherapy was determined as relative decrease of LVEF > 5% in this study. An optimal cut off value of a cumulative total doxorubicin dose for predicting LV dysfunction, determined by means of receiver-operator characteristics curve analysis, in patients with more than any of four risk factors was lower than that in those without (> 180 mg/m2 vs. > 280 mg/m2).
Discussion
The findings of our study demonstrate that there was an association between treatment with anthracyclines, trastuzumab, or both, of patients with breast cancer and the development of LV dysfunction following chemotherapy. This association became stronger with an increase in the number of risk factors, and was especially strong for patients treated with chemotherapy who had more than four risk factors. In addition, this finding can affect an optimal cut off value of a cumulative total doxorubicin dose for predicting LV dysfunction.
Risk stratification for subsequent LV dysfunction after chemotherapy
Current advances in various cancer treatment have resulted in significant improvement in cancer-specific survival. In addition, mortality rates for breast cancer, as for other cancers, have also declined. The survival of breast cancer patients depends on many factors, including age at diagnosis, tumor stage, tumor grade, estrogen receptor status, progesterone receptor status, socioeconomic factors and other non-cancer related clinical conditions such as preexisting health status, functional status and social connections [8, 9]. Elderly patient with breast cancer in particular have been evidenced as a significantly greater risk of cardiac dysfunction after chemotherapy [10,11,12]. This finding does not apply only to breast cancer, however. Survivors of various other cancers are increasingly subject to some cardiovascular diseases related to chemotherapies, compounded by the development or progression of age-related cardiovascular risk factors with prolonged survival. The proportional distribution of cumulative causes of death for all cancer patients depends on the length of follow-up time. In the first 5 to 10 years following breast cancer diagnosis, breast cancer was found to be the cumulative primary cause of death, whereas cardiovascular diseases became the cumulative primary cause of death with longer follow-ups [6]. Among these diseases, cardiovascular toxicity remains a devastating complication of cancer treatment. The mortality rate for patients with CTRCD is as high as 60% by 2 years after treatment, representing a 3.5-fold greater hazard when compared with patients with idiopathic dilated cardiomyopathy [4]. Despite their well-documented cardiotoxicity, predominantly evidenced by LV dysfunction, anthracyclines remain the preferred agents for a wide variety of malignancies including breast cancer. The addition of trastuzumab, a monoclonal antibody directed against human epidermal growth factor receptor 2, to standard adjuvant chemotherapy significantly improves disease-free and overall survival in the 20–30% of patients with breast cancer who overexpress this proto-oncogene [13]. However, trastuzumab is also cardiotoxic, resulting in cardiac dysfunction in 3–5% of patients [13]. For patients with breast cancer, therefore, the sequential or concurrent use of two different types of agents such as anthracyclines and trastuzumab may increase myocardial injury and CTRCD is often the result of the combined detrimental effect of the two therapies.
Although early detection of CTRCD is essential for delaying progression to HF, risk stratification for the development of CTRCD before chemotherapy can be difficult. The current position paper from the ESC lists several pre-chemotherapy risk factors for the development of CTRCD. In addition, we could show that there is an association between patients with breast cancer and the development of LV dysfunction following chemotherapy. This association became stronger as the number of risk factors increased and was especially strong for patients with more than four risk factors.
Clinical implications
Risk stratification for the development of CTRCD appears to hold promise for delaying progression to HF for cancer patients with preserved LVEF who are scheduled to undergo chemotherapy. There has been a growing interest in early detection of CTRCD by means of GLS, because it is a more sensitive and robust parameter for detecting subclinical LV dysfunction than other conventional LV functional parameters [2, 14,15,16,17,18]. Specifically, the current position paper from the ESC states that a ≥ 15% early reduction in GLS during chemotherapy appears to be the most useful parameter for the prediction of cardiotoxicity, and possibly of risk of CTRCD [2]. Moreover, our group recently reported that baseline GLS was found to be associated with LV dysfunction after anthracycline chemotherapy and the development of HF during long-term follow-up of patients with malignant lymphoma and preserved LVEF [19]. Although GLS is an increasingly widely accepted perceptive parameter, it is still not a routine echocardiographic parameter in many institutions, and a number of oncologists may not familiar with it. When this technology may therefore not be available, assessment of the aforementioned clinical risk factors for the prediction of cardiotoxicity is simple and easy to use and could be useful for predicting LV dysfunction following chemotherapy. Thus, watchful observation during and after chemotherapy or early preventive strategies with established cardioprotective medications such as angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, and beta-blockers may be advisable for patients with breast cancer and preserved LVEF and with the multiple risk factors detailed in the current study report. In addition, a cumulative total doxorubicin dose is thought to be an important risk factor among the baseline risk factors for CTRCD. Our study showed the different cutoff value between patients with more than any of four risk factors and those without. Our finding suggested that even lower cumulative total doxorubicin dose can develop LV dysfunction in patients with more than any of four risk factors. Thus, attending physicians should take particular care to a cumulative total doxorubicin dose for avoid the development of CTRCD in patients with more than any of four risk factors.
Study limitations
This study comprised a small number of patients and was a single-center retrospective study, so that future prospective studies with larger patient populations will be needed to validate our findings. In addition, each risk factor was assumed to be equivalent in this study, but may in fact differ in the ability to predict the development of CTRCD.
Conclusions
This study found that breast cancer and multiple clinical risk factors were associated with LV dysfunction following chemotherapy. Our findings can thus be expected to have clinical implications for better management of patients with breast cancer referred for chemotherapy.
References
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49:1374–1403
Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, Authors/Task Force M, Guidelines ESCCfP (2016) 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). Eur Heart J 37:2768–2801
Ewer MS, Ewer SM (2015) Cardiotoxicity of anticancer treatments. Nat Rev Cardiol 12:620
Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK (2000) Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 342:1077–1084
Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C, Cipolla CM (2010) Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 55:213–220
Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD (2011) Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res 13:R64
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1–39 (e14)
Jatoi I, Chen BE, Anderson WF, Rosenberg PS (2007) Breast cancer mortality trends in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol 25:1683–1690
Dunnwald LK, Rossing MA, Li CI (2007) Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 9:R6
Serrano JM, Gonzalez I, Del Castillo S, Muniz J, Morales LJ, Moreno F, Jimenez R, Cristobal C, Graupner C, Talavera P, Curcio A, Martinez P, Guerra JA, Alonso JJ (2015) Diastolic dysfunction following anthracycline-based chemotherapy in breast cancer patients: incidence and predictors. Oncologist 20:864–872
Yu AF, Yadav NU, Eaton AA, Lung BY, Thaler HT, Liu JE, Hudis CA, Dang CT, Steingart RM (2015) Continuous trastuzumab therapy in breast cancer patients with asymptomatic left ventricular dysfunction. Oncologist 20:1105–1110
Advani PP, Ballman KV, Dockter TJ, Colon-Otero G, Perez EA (2016) Long-term cardiac safety analysis of NCCTG N9831 (Alliance) Adjuvant Trastuzumab Trial. J Clin Oncol 34:581–587
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–1672
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhaes A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P (2014) Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 27:911–939
Negishi K, Negishi T, Haluska BA, Hare JL, Plana JC, Marwick TH (2014) Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur Heart J Cardiovasc Imaging 15:324–331
Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH (2013) Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr 26:493–498
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH (2014) Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 63:2751–2768
Negishi T, Negishi K (2018) Echocardiographic evaluation of cardiac function after cancer chemotherapy. J Echocardiogr 16:20–27
Hatazawa K, Tanaka H, Nonaka A, Takada H, Soga F, Hatani Y, Matsuzoe H, Shimoura H, Ooka J, Sano H, Mochizuki Y, Matsumoto K, Hirata KI (2018) Baseline global longitudinal strain as a predictor of left ventricular dysfunction and hospitalization for heart failure of patients with malignant lymphoma after anthracycline therapy. Circ J 82:2566–2574
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamashita, K., Tanaka, H., Hatazawa, K. et al. Association between clinical risk factors and left ventricular function in patients with breast cancer following chemotherapy. Int J Cardiovasc Imaging 37, 197–205 (2021). https://doi.org/10.1007/s10554-020-01976-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-01976-5