Abstract
Three-dimensional (3D) speckle tracking echocardiography (STE) is a reliable clinical tool for accurate measurements of left ventricular (LV) volumes and ejection fraction (EF). In this prospective study, we sought to identify an association between 3DSTE longitudinal strain abnormalities and areas of late gadolinium enhancement (LGE). In 50 patients (52 ± 18.5 years old) referred to our hospital for clinically indicated CMR, 3D full-volume trans-thoracic acquisitions on apical views were performed within 1 h of CMR, in order to obtain LV volumes and ejection fraction as well as global and segmental peak systolic longitudinal strain. Relative amount of fibrosis was defined based on LGE CMR with grey-scale threshold of 5 standard deviations above the mean signal intensity of the normal remote myocardium. We found a moderate positive correlation between global longitudinal strain (GLS) by 3DSTE and LGE proportion (r = 0.465, p = 0.001). The area under the receiver operating characteristic curve was 0.79. In addition, abnormal GLS could detect LGE-determined myocardial fibrosis with a sensitivity of 84.6%, a specificity of 84.8%, a positive predictive value of 69% and negative predictive value of 93%, considering an optimal GLS cut-off value of − 15.25%. Regarding 3DSTE capacity of localizing segmental LGE involvement, about 70% of LGE-positive segments presented a concomitant longitudinal strain reduction. This prospective study shows that 3DSTE longitudinal deformation is moderately associated with the extent of myocardial fibrosis, with a promising potential role in ruling out prognostically relevant fibrosis as detected by LGE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Three-dimensional (3D) speckle tracking echocardiography (STE) is a novel clinical tool that has been shown to accurately evaluate left ventricular (LV) volumes and systolic function in terms of ejection fraction (EF) and strain analysis (global and segmental) in different patient populations [1–6].
Although two-dimensional (2D) speckle tracking strain analysis has been demonstrated to detect subtle subclinical myocardial damage earlier than other conventional echocardiographic techniques [7–12], it has not been fully integrated in clinical routine practice due to its significant limitations (time-consuming analysis, out-of-plane motion of speckles).
The gold standard in clinical practice for “in-vivo” tissue characterization is late gadolinium enhancement (LGE) detected by cardiac magnetic resonance (CMR). Indeed, it has been shown to be a reliable method for detecting and quantifying myocardial fibrosis, a pathological expansion of the myocardial interstitium which is a common manifestation of most advanced cardiomyopathies with major prognostic implications [13–15]. A few studies have already demonstrated an association between 2D longitudinal segmental strain with areas of LGE by CMR [16, 17] but the long time required for the 2D strain analysis has limited its clinical applicability. The advent of 3DSTE allowed overcoming 2D-strain-analysis limitations; nevertheless, the lack of clear normal ranges of strain parameters has traditionally precluded their widespread use in the clinical arena. Recent multicentre studies have clearly defined normal reference values of LV strain parameters using 3DSTE in a large healthy population [18, 19], thus making this tool more applicable in the clinical practice, especially considering its much shorter time of analysis over 2D STE (around one-third of 2D STE time) [20].
In this study we sought to identify an association between 3DSTE longitudinal strain abnormalities and areas of late gadolinium enhancement (LGE) detected by CMR in a heterogeneous patient population of all-comers.
Methods
Study design
We prospectively recruited consecutive patients who underwent clinically-indicated CMR and agreed to undergo echocardiography on the same day. Exclusion criteria were: age < 18 years, irregular heart rhythm (such as atrial fibrillation or frequent premature beats) during scan, poor echocardiographic window, acute cardiac symptoms (during the 3 months before CMR) suggesting possible acute cardiac damage (i.e. acute chest pain, dyspnoea). Two patients were screened but not enrolled in the study due to poor echocardiographic window.
All study patients underwent a transthoracic echocardiogram with a proper three-dimensional probe and a CMR in the same day within 1 h. Echo and CMR analyses were performed blinded.
3D echocardiography
Four- or six-beat full-volume 3D LV data sets were obtained during breath-hold from apical view, using Philips iE33 (Philips Medical Systems, the Netherlands) equipped with a 3D trans-thoracic probe (X3-1 transducer). The mean temporal resolution of 3D data sets was 34 ± 9 volumes/sec (range 22–55 volumes/sec).
The 3D data sets were exported in Vol DICOM format to separate workstation equipped with the vendor-independent software package 4D LV-Analysis© 3.0 (TomTec Imaging Systems GmbH, Unterschleissheim, Germany). LV analysis were performed offline by an experienced reader blinded to CMR results. The software 4D LV-Analysis provides a step by step workflow: (1) View alignment with the manual adjustment of automatically detected axes, (2) Beutel revision with the manual adjustment of automatically generated green contours to produce a static 3D LV surface model, (3) Tracking revision with the manual adjustment of automatically generated green contours to produce a dynamic 3D LV surface model; (4) Analysis with dynamic and static maps and time curves. LV volumes were semi-automatically computed to obtain End Diastolic Volume (EDV), End Systolic Volume (ESV) and Ejection Fraction (EF). Three-dimensional speckle-tracking-derived Global Longitudinal Strain (GLS) and peak systolic Longitudinal Strain (psLS) for 16 standard American Heart Association myocardial segments, were obtained from apical views based on detection of natural acoustic speckles. The latter are acoustic markers within the myocardium that can be followed frame-by-frame during several consecutive images regardless of cardiac inevitable motion. The software calculates mean strain values for whole predefined LV segments thus generating segmental curves whose peaks values are recorded as psLS.
The percentage of myocardial segments with positive LGE and overlapped abnormal segmental psLS was calculated. Peak systolic LS was defined “abnormal” at the cut-off value of + 1 SD from the mean of age/sex-related normal reference values for segmental specific location (basal, middle, apical), in accordance to recent studies assessing normal reference values for 3DST strain18. Indeed, differences in the magnitude of LV strain are present between different age and sex groups, as well as between different segments, walls. Therefore, the functional non-uniformity of the normal LV requires the use of segment-specific normal ranges for longitudinal strains.
In 10 randomly selected patients, intraobserver and interobserver variabilities in the analysis of LV volumes and of longitudinal strain were evaluated (Table 1). The absolute error was calculated as the absolute difference between the 2 readings; the relative error was calculated as the absolute difference divided by the mean of the two readings.
CMR
The CMR studies were performed on a 1.5 T whole-body scanner (Achieva; Philips Medical Systems, Best, the Netherlands) equipped with a five-channel cardiac phased-array coil (SENSE Cardiac, Philips Medical System), using ECG-gating. Standard steady-state free precession cine imaging with full ventricular coverage was performed. Late Gadolinium Enhancement (LGE) imaging was carried out 10 min after intravenous injection of 0.15 mmol/kg body weight Gadobutrol (Gadovist; Bayer Schering Pharma, Schering, Berlin, Germany) in the same short-axis orientation with full LV coverage using a three-dimensional inversion-prepared spoiled gradient echo sequence. CMR data were analysed using a commercial image viewing software (IntelliSpace Portal v.7, Philips Healtcare, Best, Netherlands) by an experienced reader blinded to the echocardiographic results. LV volumes and EF were assessed from the cine short-axis stack. LGE presence was visually assessed in each segment using the 16-segment cardiac model. LGE total amount was assessed semi-automatically: epicardial and endocardial contours as well as a region of interest (ROI) within remote myocardium were traced manually in each slice. Areas of LGE were defined as a signal intensity of more than 5 standard deviations (SD) above remote myocardium. LGE total amount was expressed as a percentage of total myocardial mass.
Statistical analysis
Statistical analysis was performed with MATLAB 2015 (The MathWorks, Inc., Natick, MA, USA). Continuous data are presented as mean ± SD and were compared using the Student t-test. Pearson’s correlation coefficient (r) was calculated to express correlation between 3DSTE and CMR measurements. Comparison between 3DSTE- with CMR-derived measurements was assessed by Bland–Altman analysis. A p value less than 0.05 was considered to be statistically significant.
Standard diagnostic accuracy tests were calculated for the assessment of the capacity of 3DST-GLS to detect myocardial fibrosis. Receiver operating characteristic (ROC) curve was generated for GLS to detect myocardial fibrosis in order to summarise accuracy over a range of different test thresholds. In addition, Cohen’s kappa (κ) coefficient was also calculated in order to take into account the inter-raters agreement occurred by chance for observed diagnostic accuracy. Cohen’s κ coefficient was calculated applying the following equation: κ = Po (observed agreement) − Pe (hypothetical probability of chance agreement)/1 − Pe.
Ethical approval
The study protocol was approved by the San Raffaele Scientific Institute Ethics Service (04/int/2015) and complied with the Declaration of Helsinki and all patients gave written informed consent.
Results
Patient characteristics
We included in the study a total of 50 patients (70% males; mean age was 52 ± 18.5 years) whose baseline characteristics are reported in Table 2. Four patients (8%) underwent CMR examination without gadolinium injection. Our study population presented LV diastolic volumes ranging from 85 to 349 ml, LV systolic volumes ranging from 21 to 262 ml and LV EF from 25 to 78%. Twenty-nine patients (63%) were LGE positive and they presented a wide range of myocardial diseases including hypertrophic cardiomyopathy (8 patients), dilated cardiomyopathy (9 patients), myocardial-infarction-related scarring (4 patients), convalescent myocarditis (5 patients), chemotherapy-induced cardiomyopathy (1 patient), valvular cardiomyopathy (1 patient), sarcoidosis (1 patient).
LV volumes and EF
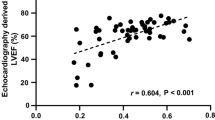
In Fig. 1 and Table 3 we report the correlation analysis between CMR and 3DSTE-derived LV volumes and EF. 3DSTE-derived parameters show good positive correlations with CMR-derived ones. The correlation for EF measurements (r = 0.902, p < 0.001) was stronger than the one regarding volumes (EDV: r = 0.783, ESV: r = 0.865; p < 0.001). In addition, 3DSTE-derived LV volumes were significantly underestimated compared with CMR with a bias of 45.83 ml for EDV and 24.8 ml for ESV. Notably, Bland–Altman plots show a proportional error: the bias presents an increasing trend with higher LV volumes. EF measurements though show a bias of −2.43%.
Correlation plots (left panel) and Bland–Altman plots (right panel) comparing 3DSTE- with CMR-derived measurements. From top to bottom depicting LVEDV, LVESV and LVEF. Bland–Altman plots visually illustrate the dispersion of 3DSTE measurements for one CMR parameter. On the y-axis the absolute difference between 3DSTE and CMR measurements is reported and on the x-axis the CMR derived value. Bias and limits of agreement are also indicated in this figure. EDV end-diastolic volumes; ESV end-systolic volumes, EF ejection fraction; r correlation coefficient
3DSTE longitudinal strain and LGE
In the study population, the mean peak global longitudinal strain GLS was − 16.6% ± 5.6% and 17 patients (34%) presented abnormal GLS values (considering the optimal cut-off generated by the ROC curve analysis). On the other hand, 29 patients (63%) were LGE positive with a mean of 9.8% ± 8.7% (range 1–29%).
Figure 2 shows a statistically significant moderate correlation between LGE burden and GLS (r = 0.465, p = 0.001). The Area Under the Curve (AUC) was 0.79. Considering the “prognostic” cut-off value of 10% LGE for definition of myocardial fibrosis [21–24], GLS detected myocardial fibrosis determined by LGE with a sensitivity of 84.6%, a specificity of 84.8% with the optimal GLS cut-off value of -15.25% as generated by ROC curve (Fig. 3). Particularly, the positive predictive value of the test was low (PPV = 68.7%) as opposed to its negative predictive value (NPV = 93.3%). Accordingly, the LGE burden in patients with normal GLS is significantly lower compared to the one of patients with abnormal GLS (3.05 ± 4.87% in nGLS vs 13.34 ± 9.9% in aGLS, p < 0.005).
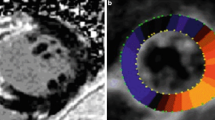
CMR LGE basal, mid-, apical slices (upper section) and 3DSTE segmental strain values reported in Bull’s eye diagram (lower-left) in the same patient. It is clear the concomitant presence of abnormal strain values in the segments presenting LGE. The table (lower-right) provides information about the diagnostic accuracy of abnormal GLS values (aGLS) in detecting LGE-determined myocardial fibrosis. GLS peak global longitudinal strain, LGE late gadolinium enhancement. *κ = Po (observed agreement) − Pe (hypothetical probability of chance agreement)/1 − Pe
When taking into account the part of agreement that may occur by chance, the agreement is still meaningful as the Cohen’s κ coefficient is 0.73.
The analysis of segmental peak longitudinal strain was used to identify the number of myocardial segments with abnormal peak longitudinal strain (considered as 1 standard deviation under the mean of normal reference for sex, age and segment) and a concomitant positive LGE by CMR. Out of 82 LGE-positive segments, 57 (69.5%) presented a concomitant longitudinal strain reduction, see Fig. 3.
Discussion
The major findings of this prospective study are that (1) there is a moderate correlation between GLS by 3DSTE and LGE detected by CMR; (2) there is a meaningful overlap between segments presenting an abnormal segmental longitudinal strain and concomitant LGE; (3) 3DSTE confirms to be a reliable diagnostic tool for calculation of LV volumes and EF even in a heterogeneous patient population of all-comers. The 3D echo-derived LV volumes are steadily underestimated, which is in keeping with previous data [25] as in most patients 3D echocardiography cannot differentiate between the myocardium and trabeculae.
LGE-determined myocardial fibrosis: a promising prognostic marker in cardiomyopathy
LGE detected by CMR offers the futuristic capacity to identify and quantify “in vivo” the burden of myocardial fibrosis, a pathological expansion of the myocardial interstitium which is a common manifestation of most advanced cardiomyopathies [13–15]. According to the concept that gadolinium is restricted to the extracellular compartment (interstitium) and excluded from normal intracellular space, contrast enhancement reliably identifies expansion of the interstitium, more commonly related as fibrosis. The introduction of LGE permitted a comprehensive “in vivo” phenotypic characterization of cardiomyopathies with major prognostic implications as many studies have demonstrated a correlation between the extent of LGE and major clinical endpoints. In particular, Bello et al. [21] have shown that ischemic patients with LGE ≥ 10% of LV mass had twofold increase risk of death and those with higher LGE burden were at even higher risk. The DETERMINE trial [22] is currently exploring potential prognostic benefits of implanting cardioverter defibrillators in ischemic patients with LGE-detected fibrosis ≥ 10% of LV mass. In another study, Gao et al. [23] demonstrated that LGE burden was significantly elevated among both ischemic cardiomyopathy and dilated cardiomyopathy patients going on to have appropriate implantable cardioverter-defibrillator therapy, sudden cardiac death, or cardiac arrest. Chan et al. [26] showed in HCM that SCD risk proportionally increased with respect to the extent of LGE, particularly beyond the cut-off of LGE > 10%. A recent meta-analysis [24] systematically reviewed a number of studies exploring the prognostic predictive power of LGE in the context of hypertrophic cardiomyopathy including 1063 patients with a mean follow-up of 3.1 years. The presence of LGE was associated with an elevated risk of cardiac death (pooled odds ratio, OR 2.9) and heart failure death (pooled OR, 5.7).
Although our study population was carefully selected for LGE to be most likely a marker of myocardial fibrosis, LGE is not a univocal marker of fibrosis as it also detects oedema, inflammation, vascularity and protein infiltration. Such factors can also be of prognostic value and they tend to be associated to abnormal strain values as well. These limitations are further discussed below.
3DST longitudinal strain and LGE-determined myocardial fibrosis
In pre-3D era, a few studies had shown an association between 2D-longitudinal segmental strain with areas of LGE by CMR in particular clinical contexts [16, 17]. In one study [18] the authors recorded decreased 2DST global longitudinal strain, even with normal left ventricular systolic function among children with acute myocarditis. In another study [19], the authors demonstrated a moderate correlation between the amount of myocardial fibrosis determined by LGE CMR and peak systolic longitudinal strain (r = 0.538, p = 0.007). Myocardial fibrosis (defined as LGE > 10%) was predicted by abnormal systolic longitudinal strain with a sensitivity of 65% and a specificity of 75% in patients with severe aortic stenosis. In further studies, 2D speckle-tracking echocardiography has been found to reflect myocardial viability and extent of necrosis in ischemic cardiomyopathy [27, 28]. Despite these encouraging data, 2D speckle tracking deformation imaging presented considerable limitations such as time-consuming acquisition and analysis (2DST, 409 ± 71 sec vs 3DST, 145 ± 26 sec) [20], out-of-plane motion of speckles thus compromising its feasibility in clinical routine.
The advent of 3DSTE together with its standardization of normal-reference values [18, 19], generated a renewed interest in exploring speckle-tracking diagnostic capacity for tissue characterization.
This is the first prospective study investigating the correlation between the extent of myocardial fibrosis (defined by LGE CMR) and myocardial deformation by 3DSTE in a heterogeneous population. Our results show a moderate association between abnormal 3DST peak global longitudinal strain and myocardial fibrosis determined by LGE (r = 0.465, p = 0.001), and the greater the extent of LGE-fibrosis, the more compromised the GLS. The ROC-related AUC of 0.79 confirms a moderate performance of the test in detecting LGE-positive area within the myocardium. Similarly, when considering the prognostic cut-off of LGE ≥ 10% of LV mass, GLS has a rather weak positive predictive value (PPV 69%). These data are in keeping with the moderate correlation already reported in previous results comparing 2DSTE vs LGE with AUC ranging from 0.358 to 0.721 [19]. Furthermore, abnormal 3DST peak longitudinal segmental strain can only moderately localize the concomitant segmental LGE involvement with about 70% of overlap.
The moderate diagnostic capacity of strain imaging in detecting and localizing myocardial fibrosis, is to be interpreted in the light of the fact that these two methods evaluate different features of myocardial fibres. While strain analysis studies the myocardial mechanical function (deformation), the LGE roughly mirrors histologic fibrosis in this context. The latter often impairs myocardial function locally thus causing an abnormal myocardial deformation. On the other hand, abnormal strain might be due to other causes than fibrotic degeneration reflecting high afterload, hypertrophy, diabetes, tobacco use, metabolic impairment or myocardial stunning. Finally, the association between 3DSTE GLS and LGE is not strong enough to propose 3D echocardiography as an alternative to CMR for tissue characterization.
Nevertheless, in our study those patients with normal GLS presented significantly lower LGE burden than those with abnormal GLS (5.5 ± 3.9% vs 14 ± 10.1% respectively, p = 0.00385). Accordingly, when considering the prognostic cut-off of ≥ 10% (LGE % of LV mass), GLS showed a high negative predictive value (NPV = 93% observed in the study) as opposed to its modest positive predictive capacity (PPV = 69%). Consequently, these findings offer a potential role for 3DSTE echocardiography in ruling out patients without “prognostically significant” myocardial fibrosis. However, after these preliminary results, further studies will be required to validate such role.
Future perspectives in myocardial tissue characterization
CMR and echocardiography are the best imaging methods to meet the need for participant safety, lack of radiation, and non-invasiveness. Traditionally, CMR has been superior in allowing complete coverage of the heart avoiding geometric assumptions, but the transition to 3D echocardiography has blunted significantly this superiority as 3D data-sets now encompass the whole heart as well. Nevertheless, CMR is still the gold standard for cardiac tissue characterization whereas 3D echocardiography does not provide sufficient insights in such regard. However, as demonstrated in our study, 3DSTE longitudinal strain is related to traditional CMR-parameters of tissue characterization (namely LGE). Theoretically, in resource-limited healthcare systems or areas with limited access to CMR, 3DSTE has a potential role in targeting CMR-contrast examinations to patients most likely to benefit from advanced tissue characterization, especially exploiting its high negative predictive value in excluding a prognostically significant LGE burden. Indeed, a CMR scanner is 4 to 10 times the cost of a standard high-quality 3D echocardiographic system, and operating costs are also higher [29, 30]. Furthermore, in some conditions where myocardial fibrosis is a progressive phenomenon (such as hypertrophic cardiomyopathy) [31], several CMR examinations may be required to follow-up the progression of the disease with a consequent further difference in cost-effectiveness between the 2 techniques. Feasibility can be an issue with both methods: patients with poor acoustic window remain challenging for echocardiography even if recent studies demonstrate that even in patients with a limited acoustic window, longitudinal strain by speckle tracking remains accurate and reproducible [32]. In our study only 2 patients were not enrolled in the study due to poor echocardiographic window. On the other hand, claustrophobia and metallic implants affect traditionally CMR feasibility. Moreover, the patients’ wish of a bed-side examination, of avoiding intravenous access and the risk of contrast-agent toxicity make 3DSTE an interesting complementary tool to CMR for tissue characterization. Finally, in a recent bi-modality study [20] the average time required for complete analysis of 3DSTE global strain measurements was significantly shorter (145 ± 26 s) compared with CMR (298 ± 64 s, P < 0.01) and 2DSTE (409 ± 71 s, P < 0.01). Nowadays, GLS parameter is easily displayed in new echocardiography machines thanks to the integrated strain-softwares without the need of exporting 3D data-set for external off-line analysis.
In Fig. 4 we propose a diagnostic algorithm for patients affected by cardiomyopathies when tissue characterization is to be investigated in the context resource-limited healthcare systems. In real-world individual hospitals, different types of imaging tests and expertise may be available; therefore, the choice of which diagnostic pathway to use is a result of a balance between accuracy, availability and cost. Our proposed algorithm is provocatively designed to express the potential complementary role of 3DSTE and to stimulate further research in order to assess the accuracy of 3DSTE strain compared with LGE in a larger population (i.e. testing optimal cut-off values derived by ROCs) and to encourage development of economic models and cost-effectiveness studies. Notably, our study population is rather heterogeneous including mainly hypertrophic cardiomyopathy, dilated cardiomyopathy, myocardial-infarction-related scarring and convalescent myocarditis. Such heterogeneity certainly supports the potential role of 3DSTE Longitudinal Strain in the assessment of fibrosis in a wide spectrum of cardiomyopathies, but, theoretically, the different LV mechanics and geometry in such distinct conditions may significantly affect the measurements of GLS regardless of fibrosis.
Proposed diagnostic algorithm for patients affected by cardiomyopathies when tissue characterization is to be investigated. This algorithm could be ideally applied in resource-limited healthcare systems or in areas with limited access to CMR, after being confirmed in larger accuracy and cost-effectiveness studies. *Proportion of patients excluded from this study. Data could vary significantly in larger studies. **Data derived from this study in predicting LGE > 10% (prognostic threshold). Data could vary significantly in larger studies
Limitations
This study presents some general limitations including the lack of detailed clinical information and parameters, the arbitrarily set grey-scale value of 5 SDs above the mean signal intensity of normal nulled myocardium for LGE and all the limitations of 3DSTE (most importantly the low temporal resolution). Furthermore, the comparison of abnormal segmental strain with concomitant segmental LGE involvement is limited by the necessity of slice-position matching, which is obviously difficult to obtain with two different imaging modalities. However, this limitation does not apply to GLS as it averages the strain values of all the LV slices acquired in the 3D data-set.
The major limitation of this study is that LGE is not a univocal marker of fibrosis as it also detects oedema and inflammation within the myocardium. Nevertheless, our population of chronic patients was selected in order to exclude patients with acute myocardial damage; therefore LGE can be reasonably interpreted as myocardial fibrosis in most cases. In this regard, the diagnosis of “convalescent myocarditis” was made when there was evidence of LGE with normalization of T2-signal (a sign accounting for oedema) as previously proposed, but not all patients underwent an endomyocardial biopsy and no record has been kept for this study. However, even in a chronic setting of cardiomyopathies, LGE should not be considered an unequivocal marker of fibrosis and this is exemplified by the global subendocardial involvement in amyloid where interstitial water is sometimes associated with amyloid fibrils rather than fibrosis [33]. Nevertheless, in our study population no patient is affected by cardiac amyloidosis and the remaining population is likely to present fibrosis within the myocardial tissue (e.i. infarct-related scarring, convalescent myocarditis, dilated and hypertrophic cardiomyopathies represent 90% of the total population).
Another possible limitation is the complex relation between strain and the heterogeneous LGE patterns in our study population (subendocardial vs mid wall vs subepicardial). Theoretically, LV longitudinal strain might be predominantly affected by subendocardial scarring and data about the relation between myocardial scar distribution and the layer-dependent multidimensional LV mechanics are limited. Nevertheless, a recent study [34] showed that 2D Speckle Tracking Longitudinal Strains are attenuated regardless of myocardial scar location. In particular, 59 patients with evidence of myocardial fibrosis on contrast-enhanced magnetic resonance imaging underwent speckle tracking echocardiography for measuring subendocardial, midmyocardial, and subepicardial strains in longitudinal direction and the authors concluded clearly that 2D Speckle Tracking Longitudinal Strains are attenuated independent of myocardial scar location.
Conclusion
This prospective study shows a moderate association between the burden of myocardial fibrosis determined by LGE and myocardial longitudinal deformation by 3DSTE in a heterogeneous study population. These data suggest that 3DSTE, beyond being a standardized tool for LV volumes and EF, has a potential diagnostic role especially in ruling out patients with prognostically relevant fibrosis as detected by LGE.
Abbreviations
- 2D:
-
Two dimensional
- 3D:
-
Three dimensional
- 2DSTE:
-
Two dimensional speckle tracking echocardiography
- 3DSTE:
-
Three dimensional speckle tracking echocardiography
- CMR:
-
Cardiac magnetic resonance
- EDV:
-
End diastolic volume
- EF:
-
Ejection fraction
- ESV:
-
End systolic volume
- GLS:
-
Global longitudinal strain
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricular
- psLS:
-
Peak systolic longitudinal strain
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- SD:
-
Standard deviations
- STE:
-
Speckle tracking echocardiography
References
Kleijn SA, Aly MF, Terwee CB, van Rossum AC, Kamp O (2012) Reliability of left ventricular volumes and function measurements using three-dimensional speckle tracking echocardiography. Eur Heart J Cardiovasc Imaging 13(2):159–168
Kleijn SA, Brouwer WP, Aly MF, Russel IK, de Roest GJ, Beek AM, van Rossum AC, Kamp O (2012) Comparison between three-dimensional speckle-tracking echocardiography and cardiac magnetic resonance imaging for quantification of left ventricular volumes and function. Eur Heart J Cardiovasc Imaging 13(10):834–839
Maffessanti F, Nesser HJ, Weinert L, Steringer-Mascherbauer R, Niel J, Gorissen W, Sugeng L, Lang RM, Mor-Avi V (2009) Quantitative evaluation of regional left ventricular function using three-dimensional speckle tracking echocardiography in patients with and without heart disease. Am J Cardiol 104(12):1755–1762
Nesser HJ, Mor-Avi V, Gorissen W, Weinert L, Steringer-Mascherbauer R, Niel J, Sugeng L, Lang RM (2009) Quantification of left ventricular volumes using three-dimensional echocardiographic speckle tracking: comparison with MRI. Eur Heart J 30(13):1565–1573
Perez de Isla L, Balcones DV, Fernandez-Golfin C, Marcos-Alberca P, Almeria C, Rodrigo JL, Macaya C, Zamorano J (2009) Three-dimensional-wall motion tracking: a new and faster tool for myocardial strain assessment: comparison with two-dimensional-wall motion tracking. J Am Soc Echocardiogr 22(4):325–330
Reant P, Barbot L, Touche C, Dijos M, Arsac F, Pillois X, Landelle M, Roudaut R, Lafitte S (2012) Evaluation of global left ventricular systolic function using three-dimensional echocardiography speckle-tracking strain parameters. J Am Soc Echocardiogr 25(1):68–79
Kim SA, Park SM, Kim MN, Shim WJ (2016) Assessment of left ventricular function by layer-specific strain and its relationship to structural remodelling in patients with hypertension. Can J Cardiol 32(2):211–216
Negishi K, Negishi T, Haluska BA, Hare JL, Plana JC, Marwick TH (2014) Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur Heart J Cardiovasc Imaging 15(3):324–331
Saccheri MC, Cianciulli TF, Lax JA, Gagliardi JA, Caceres GL, Quarin AE, Kisinovsky I, Rozenfeld PA, Reisin RC, Aadelfa (2013) Two-dimensional speckle tracking echocardiography for early detection of myocardial damage in young patients with Fabry disease. Echocardiography 30(9):1069–1077
Tan TC, Bouras S, Sawaya H, Sebag IA, Cohen V, Picard MH, Passeri J, Kuter I, Scherrer-Crosbie M (2015) Time trends of left ventricular ejection fraction and myocardial deformation indices in a cohort of women with breast cancer treated with anthracyclines, taxanes, and trastuzumab. J Am Soc Echocardiogr 28(5):509–514
Wang Q, Gao Y, Tan K, Li P (2015) Subclinical impairment of left ventricular function in diabetic patients with or without obesity: A study based on three-dimensional speckle tracking echocardiography. Herz 40(Suppl 3):260–268
Yamamoto T, Tanaka H, Matsumoto K, Lee T, Awano H, Yagi M, Imanishi T, Hayashi N, Takeshima Y, Kawai H, Kawano S, Hirata K (2013) Utility of transmural myocardial strain profile for prediction of early left ventricular dysfunction in patients with Duchenne muscular dystrophy. Am J Cardiol 111(6):902–907
White JA, Patel MR (2007) The role of cardiovascular MRI in heart failure and the cardiomyopathies. Magn Reson Imaging Clin N Am 15(4):541–564
Stirrat J, White JA (2013) The prognostic role of late gadolinium enhancement magnetic resonance imaging in patients with cardiomyopathy. Can J Cardiol 29(3):329–336
American College of Cardiology Foundation Task Force on Expert Consensus D, Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch-Herold M, Kramer CM, Manning WJ, Patel M, Pohost GM, Stillman AE, White RD, Woodard PK (2010) ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 121(22):2462–508.
Uppu SC, Shah A, Weigand J, Nielsen JC, Ko HH, Parness IA, Srivastava S (2015) Two-dimensional speckle-tracking-derived segmental peak systolic longitudinal strain identifies regional myocardial involvement in patients with myocarditis and normal global left ventricular systolic function. Pediatr Cardiol 36(5):950–959
Hoffmann R, Altiok E, Friedman Z, Becker M, Frick M (2014) Myocardial deformation imaging by two-dimensional speckle-tracking echocardiography in comparison to late gadolinium enhancement cardiac magnetic resonance for analysis of myocardial fibrosis in severe aortic stenosis. Am J Cardiol 114(7):1083–1088
Kleijn SA, Pandian NG, Thomas JD, Perez de Isla L, Kamp O, Zuber M, Nihoyannopoulos P, Forster T, Nesser HJ, Geibel A, Gorissen W, Zamorano JL (2015) Normal reference values of left ventricular strain using three-dimensional speckle tracking echocardiography: results from a multicentre study. Eur Heart J Cardiovasc Imaging 16(4):410–416
Muraru D, Cucchini U, Mihaila S, Miglioranza MH, Aruta P, Cavalli G, Cecchetto A, Padayattil-Jose S, Peluso D, Iliceto S, Badano LP (2014) Left ventricular myocardial strain by three-dimensional speckle-tracking echocardiography in healthy subjects: reference values and analysis of their physiologic and technical determinants. J Am Soc Echocardiogr 858–871(8):e1
Obokata M, Nagata Y, Wu VC, Kado Y, Kurabayashi M, Otsuji Y, Takeuchi M (2016) Direct comparison of cardiac magnetic resonance feature tracking and 2D/3D echocardiography speckle tracking for evaluation of global left ventricular strain. Eur Heart J Cardiovasc Imaging 17(5):525–532
Bello D, Einhorn A, Kaushal R, Kenchaiah S, Raney A, Fieno D, Narula J, Goldberger J, Shivkumar K, Subacius H, Kadish A (2011) Cardiac magnetic resonance imaging: infarct size is an independent predictor of mortality in patients with coronary artery disease. Magn Reson Imaging 29(1):50–56
Kadish AH, Bello D, Finn JP, Bonow RO, Schaechter A, Subacius H, Albert C, Daubert JP, Fonseca CG, Goldberger JJ (2009) Rationale and design for the Defibrillators to Reduce Risk by Magnetic Resonance Imaging Evaluation (DETERMINE) trial. J Cardiovasc Electrophysiol 20(9):982–987
Gao P, Yee R, Gula L, Krahn AD, Skanes A, Leong-Sit P, Klein GJ, Stirrat J, Fine N, Pallaveshi L, Wisenberg G, Thompson TR, Prato F, Drangova M, White JA (2012) Prediction of arrhythmic events in ischemic and dilated cardiomyopathy patients referred for implantable cardiac defibrillator: evaluation of multiple scar quantification measures for late gadolinium enhancement magnetic resonance imaging. Circ Cardiovasc Imaging 5(4):448–456
Green JJ, Berger JS, Kramer CM, Salerno M. (2012) Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 5(4):370–377
Mor-Avi V, Jenkins C, Kuhl HP, Nesser HJ, Marwick T, Franke A, Ebner C, Freed BH, Steringer-Mascherbauer R, Pollard H, Weinert L, Niel J, Sugeng L, Lang RM (2008) Real-time 3-dimensional echocardiographic quantification of left ventricular volumes: multicenter study for validation with magnetic resonance imaging and investigation of sources of error. JACC Cardiovasc Imaging 1(4):413–423
Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, Udelson JE, Rowin E, Lombardi M, Cecchi F, Tomberli B, Spirito P, Formisano F, Biagini E, Rapezzi C, De Cecco CN, Autore C, Cook EF, Hong SN, Gibson CM, Manning WJ, Appelbaum E, Maron MS (2014) Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 130(6):484–495
Becker M, Lenzen A, Ocklenburg C, Stempel K, Kuhl H, Neizel M, Katoh M, Kramann R, Wildberger J, Kelm M, Hoffmann R (2008) Myocardial deformation imaging based on ultrasonic pixel tracking to identify reversible myocardial dysfunction. J Am Coll Cardiol 51(15):1473–1481
Mollema SA, Delgado V, Bertini M, Antoni ML, Boersma E, Holman ER, Stokkel MP, van der Wall EE, Schalij MJ, Bax JJ (2010) Viability assessment with global left ventricular longitudinal strain predicts recovery of left ventricular function after acute myocardial infarction. Circ Cardiovasc Imaging 3(1):15–23
Marwick TH, Neubauer S, Petersen SE (2013) Use of cardiac magnetic resonance and echocardiography in population-based studies: why, where, and when? Circ Cardiovasc Imaging 6(4):590–596
Campbell F, Thokala P, Uttley LC, Sutton A, Sutton AJ, Al-Mohammad A, Thomas SM (2014) Systematic review and modelling of the cost-effectiveness of cardiac magnetic resonance imaging compared with current existing testing pathways in ischaemic cardiomyopathy. Health Technol Assess (Rockv) 18(59):1–120
Todiere G, Aquaro GD, Piaggi P, Formisano F, Barison A, Masci PG, Strata E, Bacigalupo L, Marzilli M, Pingitore A, Lombardi M (2012) Progression of myocardial fibrosis assessed with cardiac magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 60(10):922–929
Macron L, Lairez O, Nahum J, Berry M, Deal L, Deux JF, Bensaid A, Dubois Rande JL, Gueret P, Lim P (2011) Impact of acoustic window on accuracy of longitudinal global strain: a comparison study to cardiac magnetic resonance. Eur J Echocardiogr 12(5):394–399
Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, Maestrini V, Barcella W, Rosmini S, Bulluck H, Sayed RH, Patel K, Mamhood S, Bucciarelli-Ducci C, Whelan CJ, Herrey AS, Lachmann HJ, Wechalekar AD, Manisty CH, Schelbert EB, Kellman P, Gillmore JD, Hawkins PN, Moon JC (2015) Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 132(16):1570–1579
Kansal MM, Panse PM, Abe H, Caracciolo G, Wilansky S, Tajik AJ, Khandheria BK, Sengupta PP (2012) Relationship of contrast-enhanced magnetic resonance imaging-derived intramural scar distribution and speckle tracking echocardiography-derived left ventricular two-dimensional strains. Eur Heart J Cardiovasc Imaging 13(2):152–158
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Spartera, M., Damascelli, A., Mozes, F. et al. Three-dimensional speckle tracking longitudinal strain is related to myocardial fibrosis determined by late-gadolinium enhancement. Int J Cardiovasc Imaging 33, 1351–1360 (2017). https://doi.org/10.1007/s10554-017-1115-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-017-1115-1