Abstract
The significance and spectrum of reduced right ventricular (RV) deformation, reported in endurance athletes, is unclear. To comprehensively analyze the cardiac performance at rest of athletes, especially focusing on integrating RV size and deformation to unravel the underlying triggers of this ventricular remodelling. Hundred professional male athletes and 50 sedentary healthy males of similar age were prospectively studied. Conventional echocardiographic parameters of all four chambers were obtained, as well as 2D echo-derived strain (2DSE) in the left (LV) and in the RV free wall with separate additional analysis of the RV basal and apical segments. Left and right-sided dimensions were larger in athletes than in controls, but with a disproportionate RA enlargement. RV global strain was lower in sportsmen (−26.8 ± 2.8% vs −28.5 ± 3.4%, p < 0.001) due to a decrease in the basal segment (−22.8 ± 3.5% vs −25.8 ± 4.0%, p < 0.001) resulting in a marked gradient of deformation from the RV inlet towards the apex. By integrating size, deformation and stroke volume, we observed that the LV working conditions were similar in all sportsmen while a wider variability existed in the RV. Cardiac remodelling in athletes is more pronounced in the right heart cavities with specific regional differences within the right ventricle, but with a wide variability among individuals. The large inter-individual differences, as well as its acute and chronic relevance warrant further investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Long-term endurance training induces an overload on the heart that leads to morphological and functional changes known as the “athlete’s heart”. Cardiac remodelling typically includes ventricular chamber enlargement, atrial dilatation and left ventricular (LV) wall thickening [1–3]. These cardiac changes were classically considered reversible with detraining and of benign course. Given that these structural changes observed in the athlete’s heart might appear similar to other known arrhythmogenic substrates, a potential link between cardiac remodelling due to chronic training and increased prevalence of arrhythmia has been suggested among the sportive population [4–7]. Therefore, characterizing cardiac adaptation to chronic training is essential in order to understand its physiology, recognize its expected development and its potential transformation into pathology. Additionally, it might be useful in monitoring the impact of training on the adaptation of the heart.
Our hypothesis is that right heart adaptation differs from that of the left chambers as the latter are able to better cope with overload and that cardiac remodelling in response to training is more pronounced in the right cavities following a specific pattern of overload. The application of modern non-invasive imaging modalities would be of help to better recognize and characterize this adaptation. Integration of deformation imaging together with chamber size assessment provides a more comprehensive way to analyze cardiac performance and to earlier detect dysfunction as compared to measures of cardiac performance only based on volume changes and ejection [8–10].
Consequently, our aim was to comprehensively evaluate cardiac structural and functional remodelling in competitive professional sportsmen with the use of deformation imaging with particular focus on the global and regional adaptation of the right ventricle (RV).
Methods
Study population
In this study, 150 male participants were prospectively evaluated: 100 professional competitive sportsmen and 50 age-matched sedentary healthy subjects.
All sportsmen were devoted to sports with a mid to high dynamic component according to the Mitchell classification [11], (soccer 58%, basketball 12%, volleyball 6%, hockey 6%, running 6%, handball 5% and others 7%). All of them had a training intensity of more than 18 h per week during at least the previous 5 years. In all, a comprehensive screening for cardiac disease was performed with a clinical questionnaire, physical exam, 12 lead ECG, stress test and echocardiogram.
Healthy sedentary controls were recruited from healthy members of hospital workers and university students with the inclusion criteria of not performing regular sport activity (less than 2 h per week). All control subjects underwent a clinical questionnaire, physical exam, ECG and echocardiogram to rule out heart disease. Written informed consent was obtained from all, in accordance to the Declaration of Helsinki and the study protocol was accepted by our institution’s ethics committee.
Echocardiographic study
A commercially available system (Vivid 7, General Electric-Vingmed, Milwaukee, WI, USA) with a 2.5 MHz phased-array transducer was used to perform a comprehensive two-dimensional echocardiogram. Images were acquired from the parasternal (long and short axis) and apical (four, three and two chambers) views. Three cardiac cycles for each acquisition were stored in a cine loop format on digital storage for later off-line analysis. All studies were acquired with the subject at rest, lying in the left lateral decubitus position. Athletes were scanned before the training session, at the beginning of the competition season.
Cardiac chamber dimensions were measured according to the standards of the American Society of Echocardiography [12] and indexed for body surface area (BSA) according to the DuBois formula [13]. Interventricular septum (IVS) and posterior wall (PW) thickness, antero-posterior diameter of left atrium and end-diastolic (LVEDd) and end-systolic (LVESd) diameter of the left ventricle (LV) were obtained from M-mode scans. Ventricular and atrial volumes and LV ejection fraction were derived from biplane Simpson’s method and LV mass was calculated using Devereux’s formula [14].
Right atrial (RA) and ventricular (RV) dimensions were evaluated with RA area/volume, RV end-diastolic basal, mid-ventricular and longitudinal diameters and fractional area change assessed from the apical four-chamber view using two-dimensional echocardiography. TAPSE (tricuspid annular plane systolic excursion) was determined from M-mode scans across the tricuspid annulus from apical views and used as a marker of RV longitudinal motion and surrogate of its contractile function.
LV and RV sphericity index was calculated by dividing the end-diastolic volume by the volume of a sphere whose diameter was derived from the major end-diastolic LV/RV long axis (LV or RV_LAX), [15] as follows: \({\text{Sphericity index}}={\text{EDV}}/{\left(4/3*\pi*\left( {\text{LV or RV}}_{\text{LAX}}/2\right) \right)}^{3}\) [16]. In order to analyze if cardiac remodelling was balanced or not, we calculated the ratio between areas and volumes of the ventricular and atrial chambers [17] LV/RV end-diastolic volume ratio, LA/RA end-diastolic area ratio, LV/LA end-diastolic volume ratio, RV/RA end-diastolic area ratio and RV/RA end-diastolic volume ratio.
Pulsed Doppler was used to measure flow in the LV and RV outflow tracts, including peak velocity, velocity–time integral (VTI), acceleration and ejection time for each outflow.
Myocardial deformation imaging
Myocardial deformation of both ventricles was quantified from two-dimensional echocardiography using speckle tracking (2D strain, EchoPac, General Electric Healthcare, Milwaukee, WI, US). For the analysis, the region of interest along the endocardial border was manually traced on a single frame two-dimensional echocardiographic image. The system automatically tracks myocardial motion that the operator can adjust if necessary. Special care was taken to acquire zoomed-in images of the RV and LV in apical views with a frame rate between 60 and 80 fps to ensure an adequate ratio of spatial/temporal resolution.
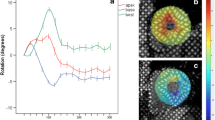
Global longitudinal strain of the LV was determined by averaging segmental myocardial strain from the four-, two- and three- chamber apical views. RV free wall longitudinal strain was measured as an average of the three free wall segments [18] (basal, mid and apical; see Fig. 1) from four chamber apical views; additionally, RV peak systolic segmental strains were measured in the basal (inlet) and apical (apical from the moderator band) segments of the RV free wall. Longitudinal strain (shortening) was expressed as negative values, with larger negative values indicating increased strain and smaller negative values indicating reduced strain.
Longitudinal strain curves in the basal (yellow trace) and apical (green trace) segments of the RV free wall calculated separately in a control subject and two athletes, as well as global longitudinal strain of the RV free wall (dotted line white trace). Note the reduced deformation values in the basal segment (smaller negative values) in athletes as compared to the control subject, particularly in athlete 2 who shows a marked difference from base to apical RV segments with a basal–apical RV strain gradient of more than 10%
Geometrical mathematical model
At rest, each individual has to generate a certain cardiac output (CO), meeting the demands of the body. At each moment, this is determined by the resting heart rate (HR) and stroke volume (SV). The stroke volume of a cardiac chamber is mathematically determined by the combination of its end-diastolic volume and the amount of (total = combined longitudinal and circumferential) strain. Output increase during exercise is then provided by changing either HR and/or SV (trough chamber size and/or chamber strain). Both the baseline ‘working point’ (a certain chamber size and strain, thus generating a defined SV), as well as which parameters change in which way, is highly individual dependent and determined by the CO demand together with the baseline cardiac size and ability to deform (i.e. normal vs damaged tissue). Therefore, we investigated this intrinsic interplay between ventricular size, strain and performance using a simplified geometrical mathematical model to determine a distribution of possible working points as well as to relate each individual to the others.
This model is described in detail in the Supplemental Digital Content (SDC). In short, three ellipsoids were used (see figure SDC1). The LV epicardium was represented by a truncated (half) ellipsoid with the two short axis radii equal. The LV endocardium was represented by the same truncated ellipsoid with reduced radii keeping the apex position fixed. For the right ventricle, given that (in normal individuals at normal pressures) it is a predominant longitudinal pump with a thin wall, we assumed no circumferential deformation so that only an endocardium was considered which is represented by half a truncated ellipsoid attached to the left ventricular epicardium, sharing the long axis. In order to obtain simplified, but physiologically plausible cardiac deformation, the following considerations have been taken into account. The outer border of the heart is constant, the LV endocardial wall contracts inwards, and the RV walls deform only longitudinal. The model was simplified to depend only on the long axis radius by measuring the average ratios of the long axis to the RV and LV (endo- and epi-) short axis in the population studied. The cardiac output was assumed equal to 5 l/min. To simulate the potential range within which the ventricles of the studied subjects could function, we calculated the size–strain-SV relations for a ventricular end-diastolic area from 15 to 50 cm2, and for SV from 20 to 130 ml. Global longitudinal strain was computed as the change in the geodesic length on the ellipsoids (see SCD).
Statistical analysis
Data were included in a database and analyzed using SPSS v.15.0 (SPSS Inc., Chicago, IL, USA). Quantitative variables are expressed as mean value ± standard deviation (SD) or median (interquartile range) when appropriate after checking for normality with the Shapiro Wilk test. Differences between groups were compared using Student’s paired T test or the U Mann-Whitney test according to normality. Qualitative variables are shown as number and percentage (%) and compared between groups using the Chi square or Fisher’s test when appropriate. Statistical significance was considered with a p value <0.05.
Results
Baseline characteristics and standard echocardiographic findings
Baseline characteristics of the study population are depicted in Table 1. All participants were males with similar age. As expected, athletes had lower heart rate and were taller and heavier; for this reason all cardiac dimensions were corrected for body surface area.
Table 2 shows standard morphological and functional parameters of left and right cavities. All end-diastolic volumes and LV mass were significantly larger in athletes as compared to those observed in controls. LV ejection fraction (LVEF) and RV fractional area change were within the normal range in both groups, despite a slight but significant reduction of LVEF in athletes (controls: 60 ± 4% vs athletes: 58 ± 4%, p < 0.05). TAPSE was also increased in athletes (24 ± 4 mm vs 26 ± 6 mm, p < 0.05).
The LV was more spherical and the RV more elongated in athletes as compared to control subjects. Additionally, the ratio between LV and RV was similar in both athletes and controls. However, the LA/RA and RA/RV size ratios were significantly different suggesting that the RA is disproportionally enlarged and unbalanced with the other cardiac chambers in athletes as compared to controls.
Outflow properties of LV and RV
Blood-pool Doppler studies confirmed the increased flows in both the RV and LV outflow tract in the athlete group as compared to the control group (Table 3). The RV outflow showed a prolonged ejection time in athletes as compared to controls. Also, the peak velocity in the RV outflow was higher in the group of athletes; however, the maximum peak was reached significantly later than in the control group, whereas in the LV outflow tract, peak velocities were higher and earlier in the athletes as compared to the control group.
Myocardial deformation imaging
Table 4 reports myocardial deformation analysis of left and right ventricles. No significant differences were found in global LV longitudinal strain (−20.1 ± 1.9% vs −19.9 ± 2.1%, n.s.) between volunteers and athletes. On average, slightly lower global RV longitudinal strain was observed at rest in athletes (−28.0 ± 3.4% vs −26.8 ± 2.8%, p < 0.05), as compared to sedentary subjects.
The regional analysis of the RV (base and apex) revealed that the significant reduction of global RV strain in the group of athletes was attributed to an important decrease in the deformation of the basal RV segment (−25.8 ± 4.0% vs −22.8 ± 3.5%, p < 0.05). The apical RV strain was similar in controls and athletes (−30.9 ± 4.5% vs −31.7 ± 5.0%), with even higher values in sportsmen.
Additionally, we identified an important subgroup of athletes who had a significant gradient in RV strain between the base and the apex: 19 athletes (21%) versus 3 volunteers (7%) (p < 0.05) had more than 15% difference in the peak systolic strain values. Figure 1 shows an example of segmental RV strain in a volunteer and in two athletes, with a marked difference in peak strain between basal and apical segments of the RV in the one on the right.
Intraobserver and interobserver intraclass correlations were performed in 10 patients and were 0.98 and 0.96, respectively, for LV global longitudinal strain; 0.93 and 0.91 for RV global longitudinal strain; 0.96 and 0.93 RV basal segmental strain; 0.92 and 0.90 for RV apical segmental strain.
Geometrical modeling
As described in the methods, an ellipsoid-based computational model was used for comparing how the LV and RV, in athletes and controls, reach their ventricular performance (i.e. which ventricular size and deformation to generate the individual stroke volume).
For model simplification, the ratio of the different axis used was calculated from the whole population. The LV long axis radius to RV short axis radius was 2.83; the LV long axis to LV endocardial short axis 1.95; and the LV long axis to LV epicardial short axis 1.36. These values were used in the formulas detailed in the Supplemental Digital Content.
Figure 2 first depicts the theoretical relationships for the LV (Fig. 2a) and the RV (Fig. 2b) by plotting the lines of constant strain in the area-SV grid. Next, the measured working points of all individuals was indicated in the plot. As can be observed, the resting working points of both ventricles were clearly different in athletes (red points) and sedentary subjects (blue circles). Additionally, a (dashed) region was indicated in which most subjects cluster for both groups. For the LV, Athletes showed a slightly more dilated LV (with still a lot of overlap with the control group), but with clearly similar LV longitudinal global strain, impendent of the size. Therefore, to cope with the higher stroke volumes, the LV dilates and deformation only increases slightly. On the other hand, the athletes’ RV dilates relatively more so that they almost fully separate on group level. However, while the smaller athlete’s RVs increase the SV by additionally increasing strain, the largest RVs are clearly working at low strain (red-filled region on Fig. 2). Therefore, RVs cope differently with increasing stroke volume, either by increasing strain and only slightly dilate or by dilating a lot but using low strain values. Thus, much more variability in the RV working point of athletes was observed with wider differences in RV strain (from −10 to −50%) than for LV strain (from −10 to −30%). This suggests a different adaptation of the RV in different individuals whereas the LV adapts progressively in all individuals.
The relationship between the parameters involved in ventricular performance: size and strain and stroke volume. The colored solid lines represent the theoretical relation of a certain strain value in the area-SV space (calculated from the ellipsoid-based computational model) for the LV (left) and the RV (right). All individuals were additionally indicated in the plots (athletes—red points; sedentary subjects—blue circles). The dashed ellipsoids indicate the regions where most subjects are located. The red semi-transparent circle indicates the aberrant RV remodeling
Discussion
This study confirms structural and functional adaptation in the heart in response to chronic endurance training, particularly involving the right cavities; in comparison to the other chambers, the RA is more dilated in athletes; the RV shows a markedly different performance in athletes as compared to sedentary subjects while the LV deformation is unaltered. Additionally, while the LV adapts similarly in most individuals, the RV shows a wider variability especially with regards to size, strain and stroke volume.
The remodelling in the athlete’s heart is predominantly right-sided
Our findings are in concordance with previous studies reporting more involvement of the RV as compared to the LV in the remodelling process associated with chronic exercise training [19, 20], with specific features resembling morphologic features of arrhythmogenic right ventricular diysplasia. Moreover, recent reports have suggested an impairment of RV systolic function in some athletes either at rest [21] or particularly after an endurance race [7, 22, 23] as demonstrated by reduced longitudinal strain of the RV. However, the interplay between chamber size and strain, both implied in the development of ventricular ejection and generation of stroke volume, was not previously analyzed. Reduced longitudinal strain may not necessarily indicate RV systolic dysfunction when analyzed isolated, as systolic ejection volume might be maintained by larger ventricular size (or increased heart rate); less strain at rest could indeed provide a larger potential contractile reserve during exercise, when the simple increase in heart rate or a slight increase in RV contractility (i.e. strain) would provide an increased stroke volume. Actually, during exercise echocardiography RV global strain proportionally increases to the increase in heart rate [24]. These different working conditions of the RV, despite not necessarily indicating RV dysfunction, might lead to higher wall stress when the ventricle is larger, and therefore, is more likely to develop myocardial fibrosis and scarring [7, 25, 26]. Finally, a wider variability in the working conditions of the RV was observed in athletes with much more uniformity in the LV performance (less dispersion in the plots relating ventricular size, strain and stroke volume). La Gerche et al. have suggested that different adaptations of the RV might be observed according to the capability of subjects to develop intrapulmonary circulatory shunting [24]. Further studies relating the presence and quantity of this shunting and RV remodelling will provide further insights into these hypothesis and observations.
Another interesting finding, suggesting a more profound remodelling of the RV as compared to the LV in athletes as a result of exercise, is the longer time required by the RV to reach the peak outflow velocity in the RV as compared to controls, whereas the LV outflow velocity remained similar. The RV took more time to eject than the LV did in athletes, an observation which was not found in untrained subjects. Both an intrinsic delay/decrease in RV contractility as well a response to an elevated RV afterload could explain these findings [23].
A disproportionate increase in RA size as compared to the other cardiac chambers was also observed in athletes and not in the control sedentary group further supporting the more important involvement of the right side in the athlete’s heart development. Indeed, it has been previously shown that both atria are dilated and potentially working at higher wall stress to cope with the increased stroke volume [9]. The proportionally more pronounced RA dilatation could be attributed to an RV contractility decrease, pressure overload and/or diastolic dysfunction.
Segmental adaptation of the RV with training
Additionally, the analysis of myocardial deformation revealed regional differences within the RV in athletes. A significant reduction of systolic strain in the basal portion of the RV free wall was observed in sportsmen as compared to sedentary subjects. This lower basal deformation of the RV is similar to those described by other authors in their group of athletes [21, 27] and its meaning remains unclear. The general population also presents a trend to have less strain in the inlet portion of the free wall of the RV [18]. This is in agreement with our control group but in athletes the fall is even greater and this decrease in deformation is not seen in the apex. Our hypothesis is that the heterogeneous morphology of the RV induced predominant damage to the basal region of the RV during exercise since the (Laplace) wall stress would be higher in the (flatter) basal segment. This seems particularly the case in some more vulnerable individuals with potentially higher local wall stresses. In this sense, we have identified a subgroup of athletes with a marked reduction of myocardial deformation in the inlet portion of the RV.
Recent studies have been published applying Tissue Doppler Imaging and speckle tracking to analyze RV performance in different states of chronic volume and pressure overload, such as atrial septal defects or pulmonary hypertension [27–30]. In patients with atrial septal defects, RV longitudinal deformation globally decreases after the closure of the defect [27, 30]. In patients with pulmonary hypertension, RV global strain is lower, but with a specific decrease in the mid-basal segments of the RV free wall [28, 29, 31].
Therefore, our findings could suggest that long-term exercise training might develop RV remodelling compatible with pressure overload in some athletes, similarly to what has been described on the right heart cavities in other experimental states of initial volume overload [32]. Classically, structural changes in the athletes’ heart have been considered secondary to volume overload induced by exercise; if such was the case, RV myocardial deformation would be similar to that observed in other situations where there is an increased circulating volume, such as septal defects (i.e. global decrease in longitudinal strain in all RV segments). However, our data demonstrated that some athletes show a pattern of myocardial deformation in the RV similar to that observed in pressure overload. The reason why some athletes develop this pattern and others do not remains to be elucidated. Unfortunately, we were not able to accurately evaluate pulmonary artery pressure because of the lack or minimal amount of tricuspid regurgitation in most studied subjects so our data are at most only hypothesis generating.
Limitations
This study only assessed deformation and size at rest. In order to get a full picture of the cardiac remodelling, this evaluation should be completed with analysis under exercise, but this is out of the scope of this study. Also, inclusion of a heterogeneous groups of sport disciplines might have different impact on cardiac adaptation but indeed, all the subjects were performing mixed trainings with most of them having high dynamic components. As opposed to previous studies [6, 7, 19–21], most of the athletes were playing team ball sports and few were endurance athletes, which might have implied different heart adaptations. Also, the current findings were observed in male athletes; further studies will have to address if they are also similar in female athletes and what the influence of gender is. Another important limitation is the lack of three-dimensional echocardiography to better estimate RV volumes and to evaluate RV shape. However, and despite its limitations, we believe that our data from two-dimensional echocardiography provides enough insights on the relative differences of the athlete’s RV as compared to the sedentary subject. On the other hand, the geometrical model, used to illustrate that the RV shown a wider spectrum of remodelling compared to the left, is based on a simplified geometry. Firstly, a constant cardiac output of 5 l/min is assumed, which is a simplification since CO will depend on body size. While this might influence size and deformation of on individual, it would not change the relative working point of the LV with regards to the RV. Geometrically, for the RV, a crescent ellipsoid might oversimplify the geometry. Additionally, the RV is modelled with only longitudinal deformation. However, young healthy individuals have predominantly longitudinal RV deformation and are not expected to have complex remodelling. Additionally, the model is used to illustrate diversity in the relations between deformation and size rather than to calculate absolute values for any variables. The current findings would probably not change significantly with a more elaborated model.
Conclusions
This study shows, by a novel comprehensive approach of integrating size and myocardial deformation, that long-term intense endurance training, in male athletes, promotes heart chamber-specific remodelling with special susceptibility of the right cavities and particularly for the inlet portion of RV and the RA. It remains to be determined whether those athletes with the more extensive remodelling are at greatest risk of arrhythmias. If such was the case, a comprehensive assessment of the cardiac performance integrating size and deformation, would be potentially useful to monitor and to stratify the risk of subjects with long term exercise training.
Clinical perspective
The changes in the morphology and function of the heart in response to chronic training have been recognized for many years and considered into the “athlete’s heart” concept, mainly characterized for the left ventricle. In recent years though, the importance of other chamber involvement such as the right ventricle (RV) and its potential relationship with arrhythmias has been highlighted. Despite being described, the characterization and the mechanisms underlying RV remodelling in response to exercise have not been well elucidated. Understanding how the RV remodels is the first step to prevent irreversible harm to the heart of athletes. In our study, we characterized the RV remodelling in athletes with a novel comprehensive approach by integrating size and myocardial deformation and analyzing segmental patterns. We observed a characteristic pattern of myocardial deformation in the RV, previously related to pressure overload. Our findings support the hypothesis of an exaggerated increase in pulmonary arterial pressure during exercise as a trigger for RV remodelling in susceptible athletes. Detecting this exaggerated response (for example with exercise echocardiography) could be useful to early identify those subjects with more risk of pronounced RV remodelling. Finally, the long term clinical implications of RV remodelling in athletes and its association with clinical arrhythmias should be further evaluated.
References
Caselli S, Di Paolo F, Psicchio C et al (2011) Three-dimensional echocardiographic characterization of the left ventricular remodelling in olympic athletes. Am J Cardiol 108:141–147
Gilbert CA, Nutter DO, Felner JM, Perkins JV, Heymsfield SB, Schlant RC (1977) Echocardiographic study of cardiac dimensions and function in the endurance-trained athlete. Am J Cardiol 40:528–533
Pluim BM, Zwinderman AH, Van der Laarse A, Van der Wall EE (2000) The athlete’s heart: a meta-analysis of cardiac structure and function. Circulation 101:336–344
Benito B, Gay-Jordi G, Serrano-Mollar A et al (2011) Cardiac arrhythmogenic remodelling in a rat model of long-term intensive exercise training. Circulation 123:13–22
Ector J, Ganame J, van der Merwe N et al (2007) Reduced right ventricular ejection fraction in endurance athletes presenting with ventricular arrhytmias: a quantitative angiographic assessment. Eur Heart J 28:345–353
Heidbüchel H, Hoogsteen J, Fagard R et al (2003) High prevalence of right ventricular involvement in endurance athletes with ventricular arrhythmias: role of an electrophysiologic study in risk stratification. Eur Heart J 24:1473–1480
La Gerche A, Burns AT, Mooney DJ et al (2012) Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J 33:998–1006
Bijnens B, Cikes M, Claus P, Sutherland G (2009) Velocity and deformation imaging for the assessment of myocardial dysfunction. Eur J Echocardiography 10:216–226
Gabrielli L, Bijnens B, Butakoff C et al (2014) Atrial functional and geometrical remodelling in highly trained male athletes: for better or worse? Eur J Appl Physiol 114:1143–1152
Rösner A, Bijnens B, Hansen M et al (2009) Left ventricular size determines tissue Doppler-derived longitudinal strain and strain rate. Eur J Echocardiogr 10:271–277
Mitchell JH, Haskell W, Snell P, Van Camp SP (2005) Task force 8: classification of sports. J Am Coll Cardiol 45:1364–1367
Lang RM, Bierig M, Devereux RB et al (2005) Recommendations for chamber quantification a report from the American Society of Echocardiography’s: guidelines and standards committee and the chamber quantification a writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18:1440–1463
Dubois D, Dubois EF (1916) A formula to estimate the approximate surface area if the height and weight be known. Arch Int Med 17:863–871
Devereux RB, Alonso DR, Lutas EM et al (1986) Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57:450–458
Mannaerts HF, van der Heide JA, Kamp O, Stoel MG, Twisk J, Visser CA (2004) Early identification of left ventricular remodelling after myocardial infarction, assessed by transthoracic 3D echocardiography. Eur Heart J 25(8):680–687
De Castro S, Pelliccia A, Casselli S et al (2006) Remodelling of the left ventricle in athlete’s heart: a three dimensional echocardiographic and magnetic resonance imaging study. Heart 92:970–976
Rudski LG, Lai W, Afilalo J et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr 23:685–713
Lang M, Badano L, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1–39
La Gerche A, Robberecht C, Kuiperi C et al (2010) Lower than expected desmosomal gene mutation prevalence in endurance athletes with complex ventricular arrhythmias of right ventricular origin. Heart 96:1268–1274
Oxborough D, Sharma S, Shave R et al (2012) The right ventricle of the endurance athlete: the relationship between morphology and deformation. J Am Soc Echocardiogr 25:263–271
Teske J, Prakken H, De Boeck B et al (2009) Echocardiographic tissue deformation imaging of right ventricular systolic function in endurance athletes. Eur Heart J 30:969–977
Neilan TG, Januzzi JL, Lee-Lewandrowski E et al (2006) Myocardial injury and ventricular dysfunction related to training levels among no elite participants in the Boston marathon. Circulation 114:2325–2333
Oxborough D, Shave R, Warburton D et al (2011) Dilatation and dysfunction of the right ventricle immediately after ultraendurance exercise exploratory insights from conventional two-dimensional and speckle tracking echocardiography. Circ Cardiovasc Imaging 4:253–263
La Gerche A, Macisaac AI, Burns AT et al (2010) Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol 109:1307–1317
Stefani L, Pedrizzetti G, De Lucca A, Mercuri R, Innocenti G, Galanti G (2009) Real-time evaluation of longitudinal peak systolic strain (speckle tracking measurement) in left and right ventricles of athletes. Cardiovasc Ultrasound 7:17
Wilson M, o′Hanlon R, Prasad S et al (2011) Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J Appl Physiol 110:1622–1626
Jate gaonkar SR, Scholtz W, Butz T, Bogunovic N, Faber L, Horstkatte D (2009)Two-dimensional strain and strain rate imaging of the right ventricle in adult patients before and after percutaneous closure of atrial septal defects. Eur J Echocardiogr 10:499–502
Dambrauskaite V, Delcroix M, Claus P et al (2007) Regional right ventricular dysfunction in chronic pulmonary hypertension. J Am Soc Echocardiogr 20:1172–1180
Kittipovanonth M, Bellavia D, Chandrasekaran K, Villaraga HR, Abraham TP, Pelikka PA (2008) Doppler myocardial imaging for early detection of right ventricular dysfunction in patients with pulmonary hypertension. J Am Soc Echocardiogr 21:1035–1041
Vitarelli A, Sardella G, Di Roma A et al (2012) Assessment of right ventricular function by three-dimensional echocardiography and myocardial strain imaging in adult atrial septal defect before and after percutaneous closure. Int J Cardiovasc Imaging 28:1905–1916
Brili S, Stamatopoulos I, Misailidou M, Chrysohoou C et al (2013) Longitudinal strain curves in the RV free wall differ in morphology in patients with pulmonary hypertension compared to controls. Int J of Cardiol 167:2753–2756
Rondelet B, Dewachter C, Kerbaul F et al (2012) Prolonged overcirculation-induced pulmonary arterial hypertension as a cause of right ventricular failure. Eur Heart J 33:1017–1026
Funding
This work was partially funded by grants from the Fundació Clinic (premio Emili Letang, B. Merino), Generalitat de Catalunya (FI-AGAUR 2014–2017, RH 040991, M. Sanz), and from the Spanish Society of Cardiology (Fundación Española del Corazón Investigación Clínica 2012), the Spanish Government (Plan Nacional I + D + i, Ministerio de Innovación y Ciencia DEP 2011–2013 (DEP 2010–20565); Intensificación Actividad Investigadora, Instituto de Salud Carlos III (M Sitges; PI11/01709); Plan Nacional I + D, Ministerio de Economia y Competitividad DEP2013-44923-P, TIN2014-52923-R and FEDER.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to be disclosed.
Additional information
Marta Sitges and Beatriz Merino equally contributed to this work and are both considered as first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sitges, M., Merino, B., Butakoff, C. et al. Characterizing the spectrum of right ventricular remodelling in response to chronic training. Int J Cardiovasc Imaging 33, 331–339 (2017). https://doi.org/10.1007/s10554-016-1014-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-1014-x