Abstract
Accurate assessment of aortic annular dimensions is essential for successful transcatheter aortic valve implantation (TAVI). Annular dimensions are conventionally measured in mid-systole by multidetector computed tomography (MDCT), echocardiography and angiography. Significant differences in systolic and diastolic aortic annular dimensions have been demonstrated in cohorts without aortic stenosis (AS), but it is unknown whether similar dynamic variation in annular dimensions exists in patients with severe calcific AS in whom aortic compliance is likely to be substantially reduced. We investigated the variation in aortic annular dimensions between systole and diastole in patients with severe calcific AS. Patients with severe calcific AS referred for TAVI were evaluated by 128-slice MDCT. Aortic annular diameter was measured during diastole and systole in the modified coronal, modified sagittal, and basal ring planes (maximal, minimal and mean diameters). Differences between systole and diastole were analysed by paired t test. Fifty-nine patients were included in the analysis. Three of the five aortic dimensions measured increased significantly during systole. The largest change was a 0.75 mm (3.4%) mean increase in the minimal diameter of the basal ring during systole (p = 0.004). This corresponds closely to the modified sagittal view, which also increased by mean 0.42 mm (1.9%) during systole (p = 0.008). There was no significant change in the maximal diameter of the basal ring or the modified coronal view during systole (p > 0.05). There is a small magnitude but statistically significant difference in aortic annulus dimensions of patients with severe AS referred for TAVI when measured in diastole and systole. This small difference is unlikely to alter clinical decisions regarding prosthesis size or suitability for TAVI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accurate assessment of aortic annular dimensions is essential for successful transcatheter aortic valve implantation (TAVI), playing a fundamental role in determining suitability for the procedure, appropriate prosthesis sizing, and minimising complications [1]. TAVI workup usually involves multimodality assessment of aortic annular size using echocardiography and angiography, with increasing use of three-dimensional imaging by multidetector row computed tomography (MDCT) [2]. However, annular measurements have been shown to vary between these modalities, which complicate assessment and may have important clinical implications for TAVI strategy [1]. Studies in animal models and humans have suggested that the aortic root is a distensible structure that exhibits dynamic changes in diameter during the cardiac cycle, with systolic and diastolic diameters varying by as much as 7.5% [3–6]. However, it is unknown whether similar dynamic variation in annular dimensions exists in patients with severe calcific aortic stenosis (AS) in whom aortic compliance is likely to be substantially reduced. Dynamic variation between systole and diastole may be clinically important since aortic annular dimensions are conventionally measured during mid systole by echocardiography and angiography, but during late diastole by MDCT [1, 7, 8]. We investigated the variation in aortic annular dimensions between systole and diastole in patients with severe calcific AS using high-resolution ECG-gated MDCT.
Methods
Subjects
Patients with severe symptomatic AS with aortic valve area (AVA) <1 cm2 or indexed AVA <0.6 cm2/m2 who underwent cardiac MDCT at our centre as part of the standard pre-procedure TAVI assessment between 2009 and 2011 were included in the study.
Pre-procedure MDCT protocol
MDCT was performed using a Siemens Definition AS+ 128 slice scanner (Siemens Medical Solutions, Erlangen, Germany). In this population with severe AS, sublingual GTN and beta-blockers were not administered specifically for the purposes of the scan. An initial topogram of the chest was performed using 120 kVp and 35 mAs. The scan of the thorax was acquired during injection of non-ionic iodinated contrast agent (Ultravist 370 Bayer Healthcare Tarrytown, New York) in an antecubital vein by a dual injector (Ultravist 370 at 6 ml/s plus a combination of Ultravist 370 and saline at the same rate). Total contrast dose was calculated individually based on patient weight (approximately 90–150 ml). Retrospectively ECG-gated data acquisition was used with 128 × 0.6 mm collimation, scan pitch of 0.18 and a gantry rotation time of 300 ms. Exposure parameters included 120 kVp tube voltage and 280–320 effective mAs, depending on patient size. Image reconstruction parameters included 180° cardiac-gated B26 (medium smooth Advanced Smoothing Algorithm) reconstruction algorithm and a temporal resolution of 150 ms. Ten cardiac phases (each 10% of RR-interval) were reconstructed with a slice thickness between 0.6 and 2 mm.
Data analysis
Images were analysed by two independent observers using the cardiac viewer application of the Vitrea Workstation (version V3.35 R006, Vital Images Inc.). Images during systole (30–40% of RR-interval) and late-diastole (70–80% of RR-interval) were loaded using slice thickness of 0.6 mm. The systolic phase was assessed by visually evaluating all phases of the cardiac cycle (0–100%) and selecting the systolic phase with maximal aortic valve leaflet opening, as described in the echocardiogram guideline for chamber quantification [9]. In our cohort this was consistently observed to be between 30 and 40% of the cardiac cycle.
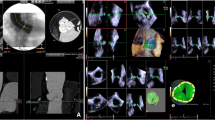
Images were reconstructed using the three multiplanar reformation planes to acquire a modified coronal and modified sagittal view and aortic annulus dimension was defined as the distance between the hinge points of the aortic valve cusps on these views as previously described [2, 10]. A transverse cut-plane at the level of the aortic valve hinge point resulted in a true double oblique transverse view of the aortic root [2]. The maximal and minimal transverse ‘basal ring’ diameters were measured at the most caudal attachments of the aortic valve as shown in Fig. 1. Additionally, we calculated aortic annulus area as previously described by Ng et al. [11] using the equation for a circular structure (πr 2). However, assuming the aortic annulus is ellipsoid in this cohort, we calculated the aortic annulus area for those patients using the equation for an ellipse area: πr 1 r 2 , where r 1 is maximal transverse basal ring diameter/2 and r 2 is minimal transverse basal ring diameter/2.
Reconstruction of a double oblique transverse image of basal ring. A vertically oriented oblique tool is placed on a coronal projection of the aortic root to produce a sagittal oblique reconstruction (a). A transverse cut-plane is placed on the sagittal reconstruction at the level of the aortic valve hinge point to create a double oblique transverse image (b). Basal ring measurements are performed at the most caudal attachments of the aortic valve (c)
Statistical analysis
Normally distributed continuous variables were expressed as mean ± standard deviation (SD). Categorical variables were presented as number and frequencies. Differences between systolic and diastolic measurements were analysed by paired sample t test. Interobserver agreements were evaluated by calculating intraclass correlation coefficients. Statistical analysis was performed with SPSS 18.0 (SPSS Inc., Chicago, IL, USA). A two sided p value of <0.05 was considered statistically significant.
Results
The study included 59 patients with severe AS (mean AVA 0.73 ± 0.2 cm2, peak gradient 82 ± 19 mmHg). The mean age was 82.4 ± 5 years with 29 (49%) male. Patient characteristics, comorbidities and scan results are presented in Table 1. Satisfactory quality systolic and diastolic phases for annular dimension assessment were obtained in all patients.
Aortic annular dimensions
The aortic annular dimensions measured in systole and diastole are summarised in Table 2. The aortic annulus was elliptical in diastole with a greater diameter in the modified coronal than the modified sagittal plane (25.5 vs. 21.6 mm, mean difference 3.9 mm), consistent with previous data from cohorts with and without AS [10, 12]. Forty-seven subjects (80%) had a ≥3 mm difference between modified coronal and modified sagittal plane diameters, which has been previously used to define an elliptical annulus [10].
Changes in annular dimensions between systole and diastole
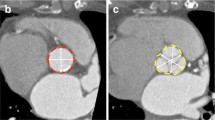
During systole there was a highly statistically significant increase in the diameters of three of the five aortic dimensions measured, as summarised in Table 2. The greatest magnitude change was a mean 0.75 mm (3.4%) increase in the minimal diameter of the basal ring during systole (p = 0.004). This plane corresponds closely to the traditional modified sagittal view (typically measured during echocardiography assessment) and accordingly a statistically significant mean 0.42 mm (1.9%) increase in that plane was also observed during systole (p = 0.008). By contrast, there was no significant change in the maximal diameter of the basal ring during systole (p = 0.16). This plane approximates most closely to the traditional modified coronal view (typically measured during angiographic assessment) and accordingly no significant change was observed that view during systole (p = 0.12). The mean basal ring diameter showed a mean 0.5 mm (2%) increase in diameter during systole (p = 0.001), which was likely to be driven by the increase in the minimal basal ring measurement. Representative examples of annular dimensions in the modified coronal, modified sagittal, and double oblique transverse views are shown in Fig. 2.
Representative example of annular dimensions in mid systole (top panel) and late diastole (bottom panel). Annular dimensions in the modified coronal view (a: 24.85 mm, d: 24.80 mm), in the modified sagittal view (b: 22.90 mm, e: 21.10 mm) and mean basal ring dimensions in the double oblique transverse view (c: 23.50 mm, f: 22.80 mm)
The small magnitude but statistically highly significant changes in the minimal basal ring and modified sagittal planes suggest subtle but consistent changes in annular shape during systole in patients with severe AS. Rather than a simple global expansion during systole, expansion in the sagittal and minimal transverse planes with little change in the coronal and maximal transverse planes suggests that the ellipticity of the annulus is reduced during systole. The mean difference between the modified coronal and modified sagittal plane diameters in individual subjects during systole was 3.2 ± 2.36 mm, compared to 3.9 ± 2.03 mm during diastole (p = 0.004). Thirty-five subjects (59%) had a ≥3 mm difference between modified coronal and modified sagittal plane diameters during systole (compared to 47 subjects (80%) during diastole). Changes in aortic annular area were consistent with these data. Annular area during diastole was 488 ± 12 mm2 and showed a small but significant increase during systole to 509 ± 12 mm2 (p = 0.002), which represents a 4% difference in area between the two phases.
Bland–Altman difference plots showing the mean differences between systolic and diastolic measurements for each aortic annulus view are illustrated on Fig. 3.
Bland-Altman plots comparing annular dimensions at late diastole and mid systole in the modified coronal view (a), modified sagittal view (b), basal ring maximal diameter (c), and basal ring minimal diameter (d). Solid lines = Mean; Dashed lines = Mean ± 2SD; LD late diastole, MS mid systole, Dmax maximal diameter, Dmin minimal diameter
Agreement with implanted CoreValve prosthesis size
Of the 59 patients included on this study, 34 had the device implanted. In this cohort, the systolic and diastolic diameter on modified coronal view correlated with the prosthesis size chosen in 32 (94%) of the patients. There was disagreement between the systolic and diastolic diameter in only 2 (6%) patients. In these cases the systolic diameter called for a small prosthesis (26 mm) and the diastolic diameter called for a large prosthesis (29 mm). Both patients had a small prosthesis implanted. The final decision about prosthesis size at our institution is made according to the angiographic measurement performed at systole in the coronal view.
Reproducibility of aortic annular measurements
MDCT-derived aortic annular dimension measurements were highly reproducible between observers in 10 randomly selected patients. The mean interobserver difference (bias) and repeatability coefficient (±2SD) was 0.011 ± 0.036. The intraclass coefficient was 0.959 (p < 0.001).
Discussion
This study demonstrates small magnitude but statistically highly significant differences in aortic annular diameter measurements during systole compared to diastole in patients with severe calcific AS undergoing workup for TAVI. The largest change was seen in the minimal basal ring plane, which demonstrated a mean increase of 0.75 mm during systole. There was no significant change in the maximal basal ring plane, suggesting that the annulus becomes more circular and less elliptical during systole.
Previous studies in experimental animal models have suggested significant dynamic changes in the aortic annulus and aortic root between systole and diastole [5, 6], but the extrapolation of these data to humans, particularly in the context of severe calcific AS is limited. Several studies using a range of imaging modalities have investigated cohorts predominantly free from significant AS, with variable findings. Transthoracic echocardiography from a cohort of 292 patients due to undergo homograft aortic valve or root replacement (75% for aortic incompetence, 25% for aortic stenosis or mixed aortic valve disease) suggested substantial aortic annular diameter changes between systole and diastole, with a mean change of 3.8% for non-calcified aortic lesions and 7.5% for normal aortic roots [4]. The haemodynamic consequences of aortic incompetence may have exaggerated the dynamic annular changes in this population. A small study using 16-slice MDCT reported no change in longitudinal dimension of the aortic annulus between diastole and systole in 25 patients free from significant aortic or valvular disease, but may have been underpowered to detect significant changes [13]. A larger study evaluating 169 patients undergoing 64-slice MDCT for investigation of coronary disease with an 11% prevalence of significant AS also reported no difference in annular diameter between diastole and systole, but the methodology used for the analysis is uncertain [10]. Mean coronal diameters were similar in systole and diastole (26.5 ± 2.9 mm and 26.3 ± 2.8 mm respectively), but the mean systolic sagittal diameter was numerically higher (24.2 ± 2.6 mm vs. 23.5 ± 2.7 mm during diastole) which is broadly consistent with our findings in patients with AS. A recent study using 64- or 256-slice MDCT in 108 subjects free of valvular or coronary disease showed a significant difference between systolic and diastolic diameters in individual subjects using a paired sample t test, but the magnitude and direction of the change were variable and there was no significant difference between mean systolic and diastolic annular dimensions of the combined cohort [3]. This highlights the importance of paired sample analysis as used in our study, but differs from the consistent change in systolic diameters that we observed in patients with AS.
Only one previous study has specifically compared annular dimensions between systole and diastole in patients with AS [12]. This examined 26 patients with severe AS referred for TAVI using 64-slice MDCT and the results are consistent with our findings in the same population. For 19 patients with paired systolic and diastolic measurements, the mean sagittal diameter was higher during systole (22.5 vs. 21.5 mm in diastole), with systolic measures larger for 11 patients, unchanged for seven patients, and smaller for one patient. As in our study, the mean coronal diameter was essentially unchanged during systole and diastole (25.6 and 25.8 mm respectively), with systolic measures larger for seven patients, unchanged for four patients, and smaller for eight patients.
Dynamic changes in aortic annular dimensions in patients with AS are important because accurate assessment of aortic annular dimensions is essential for optimal patient selection for TAVI, appropriate prosthesis sizing, and minimising complications such as paravalvular regurgitation [1]. This is particularly relevant because multimodality imaging is usually performed during workup for TAVI. Echocardiographic, angiographic and MDCT measurements are conventionally made during mid-systole, when the valve is open making the annular insertion easier to define [1, 7, 8]. This is important especially for LVOT measurements, to avoid tears during TAVI procedure and AV balloon valvulosplaty. MDCT measurements may be performed also during late diastole, when there is minimal cardiac movement and the motion artefacts are reduced, facilitating imaging analysis [1]. As previously described and confirmed in this study, the aortic annulus is elliptical rather than circular, with the consistent pattern of the sagittal diameter being smaller than the coronal diameter. Our data suggest that the shape of the annulus changes during systole with an increase in the sagittal but not the coronal diameter, resulting in the annulus tending to become more circular. The small magnitude of the change along with the fact that annular dimensions do not change uniformly in all planes during systole may in part explain the lack of difference observed in some previous studies. This pattern might also be more prominent in patients with severe AS than in other populations.
Although statistically significant, the absolute difference in annular dimensions between systole and diastole is generally small (mean 0.42–0.75 mm). The small magnitude of annular distension in this cohort is perhaps unsurprising as the molecular composition of the aorta changes during aging, with alterations in collagen fibres that become thicker and denser, losing their predominantly circumferential orientation [14, 15]. These changes result in increased stiffness of the aortic root and aorta, with the earliest and greatest effects noted in the proximal aorta [16]. Significant calcification of the valve leaflets and aortic annulus in severe AS is also likely to directly reduce annular distensibility.
The study has two main clinical implications. First, with respect to selection of patients for TAVI and appropriate prosthesis sizing, absolute differences of the magnitude we observed (<1 mm) between systole and diastole are unlikely to substantially impact on patient and device selection, particularly in the setting of multimodality imaging. However, it should be recognised that this effect may contribute to cases where the results of imaging appear discordant. Second, the results found in our study probably would not change the acquisition protocol used for this cohort. Retrospective gating appears to be more adequate due to the high incidence of arrhythmia in these patients and betablockers are not used to control heart rate at the time of the scan. In our study, the mean radiation dose was 15 mSv, probably related to a high incidence of atrial fibrillation (37.3%). Advances in scanner technology and improved imaging protocols will allow imaging at lower effective radiation doses in the future, including volume imaging, high-pitch ECG-triggered helical acquisition and novel image reconstruction [16].
Limitations
This is a single centre observational study including a relatively small number of patients that may not be a representative sample of the wider TAVI population. Secondly, we only measured the aortic annulus diameter in two phases of the cardiac cycle, and our results could be confounded by any potential co-registration error. Although these are the phases most frequently used for this purpose, a comparison of the dynamic changes in each 10% of the RR-interval may provide more complete information.
Conclusion
There is a small magnitude but statistically significant difference in aortic annulus dimensions of patients with severe AS referred for TAVI when measured in diastole and systole. This small difference is unlikely to alter clinical decisions regarding prosthesis size or suitability for TAVI.
References
Messika-Zeitoun D, Serfaty JM, Brochet E, Ducrocq G, Lepage L, Detaint D, Hyafil F, Himbert D, Pasi N, Laissy JP, Iung B, Vahanian A (2010) Multimodal assessment of the aortic annulus diameter: implications for transcatheter aortic valve implantation. J Am Coll Cardiol 55(3):186–194. doi:10.1016/j.jacc.2009.06.063
Leipsic J, Gurvitch R, Labounty TM, Min JK, Wood D, Johnson M, Ajlan AM, Wijesinghe N, Webb JG (2011) Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging 4(4):416–429. doi:10.1016/j.jcmg.2011.01.014
de Heer LM, Budde RP, Mali WP, de Vos AM, van Herwerden LA, Kluin J (2011) Aortic root dimension changes during systole and diastole: evaluation with ECG-gated multidetector row computed tomography. Int J Cardiovasc Imaging. doi:10.1007/s10554-011-9838-x
Yankah AC, Klose H, Musci M, Siniawski H, Hetzer R (2001) Geometric mismatch between homograft (allograft) and native aortic root: a 14-year clinical experience. Eur J Cardiothorac Surg 20(4):835–841
Dagum P, Green GR, Nistal FJ, Daughters GT, Timek TA, Foppiano LE, Bolger AF, Ingels NB Jr, Miller DC (1999) Deformational dynamics of the aortic root: modes and physiologic determinants. Circulation 100(19 Suppl):II54–II62
Lansac E, Lim HS, Shomura Y, Lim KH, Rice NT, Goetz W, Acar C, Duran CMG (2002) A four-dimensional study of the aortic root dynamics. Eur J Cardiothorac Surg 22:497–503
Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M (2009) Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 22(1):1–23; quiz 101–102. doi:10.1016/j.echo.2008.11.029
Kurra V, Kapadia SR, Tuzcu EM, Halliburton SS, Svensson L, Roselli EE, Schoenhagen P (2010) Pre-procedural imaging of aortic root orientation and dimensions: comparison between X-ray angiographic planar imaging and 3-dimensional multidetector row computed tomography. JACC Cardiovasc Interv 3(1):105–113. doi:10.1016/j.jcin.2009.10.014
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18(12):1440–1463. doi:10.1016/j.echo.2005.10.005
Tops LF, Wood DA, Delgado V, Schuijf JD, Mayo JR, Pasupati S, Lamers FP, van der Wall EE, Schalij MJ, Webb JG, Bax JJ (2008) Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. JACC Cardiovasc Imaging 1(3):321–330. doi:10.1016/j.jcmg.2007.12.006
Ng AC, Delgado V, van der Kley F, Shanks M, van de Veire NR, Bertini M, Nucifora G, van Bommel RJ, Tops LF, de Weger A, Tavilla G, de Roos A, Kroft LJ, Leung DY, Schuijf J, Schalij MJ, Bax JJ (2010) Comparison of aortic root dimensions and geometries before and after transcatheter aortic valve implantation by 2- and 3-dimensional transesophageal echocardiography and multislice computed tomography. Circ Cardiovasc Imaging 3(1):94–102. doi:10.1161/CIRCIMAGING.109.885152
Wood DA, Tops LF, Mayo JR, Pasupati S, Schalij MJ, Humphries K, Lee M, Al Ali A, Munt B, Moss R, Thompson CR, Bax JJ, Webb JG (2009) Role of multislice computed tomography in transcatheter aortic valve replacement. Am J Cardiol 103(9):1295–1301. doi:10.1016/j.amjcard.2009.01.034
Kazui T, Izumoto H, Yoshioka K, Kawazoe K (2006) Dynamic morphologic changes in the normal aortic annulus during systole and diastole. J Heart Valve Dis 15(5):617–621
Grande KJ, Cochran RP, Reinhall PG, Kunzelman KS (1999) Mechanisms of aortic valve incompetence in aging: a finite element model. J Heart Valve Dis 8(2):149–156
Greenwald SE (2007) Ageing of the conduit arteries. J Pathol 211(2):157–172. doi:10.1002/path.2101
Nelson AJ, Worthley SG, Cameron JD, Willoughby SR, Piantadosi C, Carbone A, Dundon BK, Leung MC, Hope SA, Meredith IT, Worthley MI (2009) Cardiovascular magnetic resonance-derived aortic distensibility: validation and observed regional differences in the elderly. J Hypertens 27(3):535–542
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bertaso, A.G., Wong, D.T.L., Liew, G.Y.H. et al. Aortic annulus dimension assessment by computed tomography for transcatheter aortic valve implantation: differences between systole and diastole. Int J Cardiovasc Imaging 28, 2091–2098 (2012). https://doi.org/10.1007/s10554-012-0018-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-012-0018-4