The development of shale gas reservoirs with water-based drilling fluids is complicated by the problem of clay mineral hydration. The method of molecular simulation is widely used in many research fields, in both humanitarian and material sciences. In this paper, based on the previously published studies, the authors propose a comprehensive review of molecular simulation of inhibiting the surface hydration swelling of clay minerals. Swelling characteristics and the adsorption properties of clay minerals are reviewed and discussed. The results can be useful for future development of the MD (molecular dynamics) simulation method and its application in studies of inhibition of the surface hydration swelling in clay minerals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Introduction
1.1. Background
The increase in the energy demand and intensive exploitation of conventional petroleum resources have shifted the focus of the oil and gas industry towards unconventional resources, shales in particular. In the oil and gas industry, 75% of all wells were drilled in shale formations, while the hydration of clay minerals in shales is responsible for 90% of the wellbore stability problems [1]. The main cause of shale instability in both soft and hard shales is the water absorption and the subsequent swelling and sloughing of the wellbore. The wellbore instability problem restricts the efficient development of unconventional petroleum resources in shales.
Hydration of the clay minerals in the formation can be effectively inhibited by the use of oil-based drilling fluids. The oil-based drilling fluids can provide good borehole stability, stable performance of the drilling fluid, and sufficient protection of the oil reservoir. The wide application of oil-based fluids is restricted by the high cost and environmental pollution caused by the fluids. Therefore, the oil-based fluids are replaced by the water-based drilling fluids with the same performance parameters [2].
The content of clay minerals in a shale formation is high. The long length of the horizontal section in a horizontal gas well results in an increased contact time of the drilling fluid with the formation, causing serious shale hydration and prominent borehole instability [3]. To prevent shale hydration, the water-based drilling fluid must provide sufficient inhibition of the surface hydration and the osmotic hydration of the clay minerals in shale. Therefore, a comprehensive understanding of the hydration swelling mechanism of clay and the mechanism of interaction between inhibitors and the clay minerals is important for providing control of the clay minerals hydration in shale on macro and micro levels.
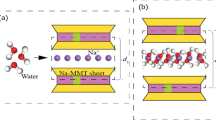
A large number of experiments and computational simulations have been conducted to understand the swelling behavior of clay systems. Two stepwise regimes of clay swelling have been determined experimentally. These are crystalline swelling and osmotic swelling. The crystalline swelling increases from the dehydrated state to the formation of the three-layer hydrates (around 19.0 Å for Na-montmorillonite). The osmotic swelling develops from a significantly larger layer spacing (around 40.0 Å for Na-montmorillonite) [4].
Clay swelling is a complex process controlled mainly by the Van der Waals and coulombic interactions between two charged layers and between the charged layers, cations, anions, and water molecules. The mechanism includes the formation of H-bonds between water molecules and clay surfaces, interactions between water molecules, and the hydration effect of cations [4]. Numerous experimental studies using the XRD, TGA, TEM, NMR, and IR methods have yielded insight into the molecular distribution and organization of the interlayer water and cations adsorbed on the mineral surfaces [5,6,7,8]. The experimental studies are focused mainly on macroscopic interactions between adsorbates and the mineral, but do not evaluate the reactions occurring at the micro-level and their mechanisms.
1.2. Introduction to molecular simulation
In the past decade, the molecular modeling methods have been increasingly applied to simulate various materials and their microstructural, physical, and thermodynamic properties [9]. The mechanism of hydration of the clay minerals at the nanoscale level is difficult to evaluate and the number of experimental and analytical studies is relatively small. Due to the complex microstructure of the layered phyllosilicate minerals and the difficulties of experimental studies at an atomic level, the method of theoretical molecular simulation can be successfully applied.
The molecular simulation method is based on experimental results and physical and chemical principles. The model and the related algorithm are constructed to calculate reasonable molecular structure and molecular behavior. The results of the simulation can be used to explain experimental phenomena and to provide guidance for further research.
Molecular simulation (MS) techniques have been involved in the study of the clay and clay-organic composites. The methods used in molecular simulation include discrete element method (DEM), Monte Carlo method (MC), molecular dynamics (MD) simulation, and quantum mechanics (QM) simulation [10]. At present, the molecular simulation method has been widely used to study the crystal structure, the hydration mechanism, and the adsorption behavior on the surface of clay minerals. The method is also applied to design and optimize reasonable and efficient clay inhibitors. In this paper, the research is based on the review of the previously published molecular simulation studies on surface inhibition and hydration expansion of clay minerals.
1.3. Advantages of molecular simulation (MS)
The molecular simulation method has a number of advantages over experimental methods when applied to hydration expansion of clay minerals. First, MS is an atomic-scale method that can be viewed as an experiment performed on a computer. The MS method can provide an atomic description of physical and/or chemical processes and interpretation of experimental results. Clay minerals usually exist in the form of ultrafine-grained crystallites ranging from micro to nanoscale, which is exactly the simulated spatial scale of the MS method. Second, our understanding of these complex processes at the atomic level is provided by a few experimental and analytical methods such as X-ray absorption and NMR spectroscopy. However, due to the complexities of structure and composition of clay and other hydrated minerals and the inherent uncertainties of experimental methods, theoretical models can be applied to provide fundamental atomic-level understanding, interpretation, and prediction of these phenomena [11]. Third, the MS method can be applied to study hydration processes under extreme conditions, including high pressure, high temperature, and extreme humidity. MS can also be used to investigate the impact of a particular factor, for example, to study the influence of the external basal surface of clay minerals on the kinetics of the hydration inhibitor. The effect of the external basal surface on the kinetics of inhibition is difficult to study experimentally because of the complex structure of clay minerals. The most important thing is that MS can provide significant insight into understanding the structures and properties of these complex phases on a molecular level.
In summary, MS can be utilized as an alternative method to replace, predict, and explain experiments by analyzing the atomic behavior in the case when the laboratory or field experiments are impossible, risky, expensive, and blind due to the limitation of the temporal-spatial scale. In this paper, the MS method is applied to study hydration and expansion of clay minerals.
2. MS application to the research of the clay minerals
Clay minerals are classified into the kaolinite group, the illite group, and the smectite group. Among these, the smectite group represents the most expandable clay minerals [12]. Smectite mineral layers have a permanent negative charge due to the isomorphous replacement of Mg2+ and Fe2+ ions for Al3+ ions in the octahedral plane or Al3+ for Si4+ in the tetrahedral plane [13]. In natural smectites, the resulting negative charge is balanced by exchangeable cations in the interlayer space between the mineral layers and the Na+, Ca2+, and other counterions. The process of swelling is caused by the counterions and their ability to adsorb water molecules.
The computer molecular simulation methods have become extremely helpful in providing information on the structure and behavior of clay minerals at the atomic level [14]. Most of the studies have been based on the classical Monte Carlo (MC) method, molecular dynamics (MD), or quantum mechanics (QM) methods. In the following sections, we review the molecular simulation of the swelling process and adsorption properties. The swelling process of clay minerals and the impact of structural and external factors are studied. The adsorption properties of clay minerals determine inhibition of the surface hydration and the osmotic hydration. In this study, the adsorption properties of three surfaces in the mineral structure are also reviewed.
2.1. MD of the swelling process
Figure 1 illustrates the basic features of the mineral structure, including basal spacing, interlayer distance, basal surface, and the edge face. The increase in basal spacing and interlayer volume is caused by hydration of exchangeable cations and migration of water molecules [15]. The process of swelling can be caused by two mechanisms - crystalline swelling and osmotic swelling. Crystalline swelling occurs with the step-wise formation of multi-layer hydrates, the number of layers varies from 1 to 4, and the basal spacing consequently increases from approximately 1.0 to 2.2 nm [16,17,18]. In the subsequent osmotic swelling, the basal spacing increases continuously rather than discretely.
The swelling behavior of smectites is affected by structural factors, including the composition and structure of clay minerals, the interlayer cation types, and the electric charge location, the solution properties, including pH, ionic strength, and ligands present in the solution, and the external factors, including temperature, pressure, and relative humidity [19,20,21,22,23,24,25,26]. In the following sections, we investigate and compare the impact of the structural factors and external factors.
2.2. MD simulations of the influence of different factors
Since the 1990s, the molecular simulation method has been applied to the research of the clay hydration process, and the results have been obtained and published. Considering the influence of structural, solution, and external factors, some authors have performed a multi-factor analysis of the swelling properties of clay minerals. In the following sections, the effects of structural factors on the swelling properties of clay minerals are reviewed.
2.2.1. Effect of the clay minerals structure
As stated previously, the swelling properties of clay minerals are determined by their structure. Many researchers compared the effect of different mineral structures on the swelling behavior. Due to its neutral charge structure, pyrophyllite is often used as a non-swelling reference mineral. For instance, the authors [27] investigated the swelling pressure of two types of interlayers and showed that the basal distance changes discretely and the pressure is lower in the pyrophyllite-montmorillonite (Mt) system than in the Mt-Mt system.
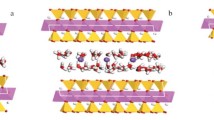
Zhan et al. [27] studied the impact of the siloxane structure on the mechanism of hydrophobic agglomeration in an aqueous solution. The kaolinite and talc minerals represent the samples with a different number of siloxane surfaces, as shown in Fig. 2. The results of the molecular dynamics simulation (MD) showed that the hydroxyl surface of kaolinite is strongly hydrophilic, while the siloxane surface of kaolinite (on the Si-tetrahedral side) and talc (both basal surfaces) show a strong hydrophobic ability due to the symmetrical siloxane structure. Therefore, the siloxane structure on the inner surfaces of minerals plays an important role in the agglomeration behavior, including the agglomeration degree and the size of the resulting flocs. The authors unveiled the correlation between the mechanism of hydrophobic agglomeration behaviors and the siloxane structure on the mineral surfaces, which has a profound significance in understanding the dispersion stability of colloidal clay minerals.
Similarly, Zhou et al. [28] compared the swelling behavior of rectorite and Mt and found that the swelling behavior of rectorite is similar to that of Mt. Due to the lower energy barrier in Mt, the swelling process goes easier from a single-layer hydrate to a double-layer hydrate than it occurs in rectorite. The local minimum of the swelling free energy means that the corresponding hydration structure is stable, while the difference between the maximum value and minimum value of the same hydration state represents the swelling energy barrier. The energy barrier for Mt is lower than that for rectorite, indicating that swelling in rectorite is harder, as shown in Fig. 3. The results of this study provide an important basis for the research and development of clay hydration inhibitors.
In the most previously published MD simulation studies, the authors assumed that the clay minerals have infinite periodicity and the adjacent ore layers are parallel. However, the actual clay is formed by the juxtaposition of fragments with different directions and contact angles. To establish a more realistic clay model, Zheng et al. [29] conducted MD simulation to study the evolution of non-parallel hydrated Na-Mt at specific temperatures (300 and 340 K) and pressures (12 and 16 MPa). The result is of great significance for the study of clay mineral expansion, as it considers the migration, rotation, and separation of the inclined mineral layers, which provides a more realistic picture. This study marks an important milestone in the MD simulation research of the swelling properties.
2.2.2. Effect of interlayer cation types
In the primary stage of opening of the interlayer space, crystalline swelling plays an important role in inhibiting the surface hydration expansion of clay minerals. Therefore, a fundamental understanding of crystalline swelling, as the effect of interlayer cation hydration on the d001-value of clay minerals, is vital for further studies of swelling.
Many scholars have investigated the impact of interlayer cation types on the swelling properties of clay minerals. The authors [30,31] studied the crystalline swelling capacity of Mt with different interlayer cation types (Na+, K+, Cs+, Ca2+, and Mg2+), and found that, due to the difference in the cation hydration strength, the crystalline swelling capacity varies in the order Ca-Mt > Na-Mt > K-Mt > Cs-Mt > Mg-Mt. J. Peng [32] obtained the same conclusion by molecular simulation.
Liu et al. [33] found that, when the water content is constant, the swelling capacity of the Ca-Mt system is lower than that of Na-Mt and K-Mt systems. Therefore, when studying the effect of interlayer cations on the swelling characteristics of clay minerals, it is important to consider the impact of other relevant factors, including humidity, clay type, temperature, and pressure parameters.
As mentioned above, the excess negative charge on the mineral surface is neutralized by the equivalent amount of positive ions. In the case of natural inhibition of surface hydration expansion, the most common exchangeable cations are Ca2+, Mg2+, H+, Na+, and K+. Therefore, to evaluate the swelling properties of clay minerals in natural clay, one needs to consider the presence of multiple cations.
Zhang et al. [34] studied the combined effect of multi-cations in comparison with the impact of a single type of cations. As shown in Figs. 4-6, the combined effect of Ca2+ and Na+ cations in Mt differs from the influence of a single type of cations. Figure 4 shows the swelling curves, the immersion energies are presented in Fig. 5, and the hydration energies are shown in Fig. 6. It can be seen, that Na+ shows a higher mobility than Ca2+, and that the Ca2+ hydration complexes demonstrate an inhibitory effect on the mobility of Na+.
Similarly, Na et al. [35] found that the replacement of a relatively small amount of interlayer Na+ cations by Mg2+ in the Na-Mt system in the initial stage of swelling can cause a substantial change in the swelling properties of interlayer cations due to the high solvation ability of Mg2+.
In some studies, the authors also considered the effect of multi-cations on the expansion properties of clays [34]. Nonetheless, further research is needed to focus on the effect of multi-cations, considering three or more cation types and their interaction in the process of expansion of clay minerals.
In recent studies, the authors have considered relatively small concentrations of Ca2+, Mg2+, H+, Na+, K+, and other exchangeable cations under different environmental conditions. To evaluate the role of cations in the swelling process, it is important to study the influence of ion concentration on the swelling behavior and the swelling properties of various clay types in inhibiting the surface hydration expansion of clay minerals.
2.2.3. Effect of the electric charge
Seppälä et al. [25] studied the effect of the layer electric charge on the swelling process and showed that when the layer charge is small, swelling is higher. The result is consistent with the results of other studies [18, 24].
Shen et al. [36] applied the molecular dynamics (MD) simulation to study the effect of charge density in montmorillonite on the hydration characteristics of the interlayer space, particularly the siloxane surface. The results show that with increase in the charge density, the hydration energy increases, and the mobility of water molecules decreases. It shows that the charge density can influence the hydrophobicity of the siloxane surface and affect the interaction with organic materials.
The authors also studied the effect of the charge location. The layer charge can be located in octahedral planes, tetrahedral planes, or both. The MD results presented by Sun et al. [37] reveal that in the case of the constant total charge, the swelling pressure increases with increase in the charge located in octahedral planes, as shown in Fig. 7. On the contrary, Liu et al. [38] found no obvious impact of the layer charge location on the swelling properties. Ngouana et al. [12] studied the effect of local charge inhomogeneity on the swelling properties and found that the specific positioning of the isomorphic substitution in the Cs-Mt structure has little effect on the thermodynamics, structure, and transport properties of the system. They predicted that the impact of the disorder of the distribution of the layer charge inhomogeneities would become prominent with an increase in the concentration of substituted sites and the total layer charge.
Compared with the other two important structural factors (i.e., the structure of clay minerals and interlayer cation types), there are relatively few studies on the influence of the total layer charge and, particularly, the layer charge location on the swelling properties of clay minerals. Therefore, the relationship between the layer charge location and the swelling properties of clay minerals needs to be further investigated.
2.3. MD simulation of adsorption properties
Clay mineral structure contains three types of surfaces - external basal surfaces, external clay edges, and interlayer basal surfaces. The surfaces are characterized by different abilities to adsorb water molecules and cations. Lammers et al. [39] found that the external basal planes show a comparatively weak selective adsorption of the Cs+ ions (i.e., a rapid ion exchange rate), while the edges and the interlayer surfaces have a much higher affinity for Cs+.
The adsorption properties of clays are strongly influenced by the following parameters: structure and composition of the clay minerals, composition and dynamics of the solution [40, 41], and structure and size distribution of the nanopores connecting clay interlayers with the inter-particle pores [42]. The ionic concentration is closely linked with the ionic adsorption because the intercalated ions are in thermodynamic equilibrium with ions in the pore water. It was shown, that with increase in ion concentration, the ion adsorption decreases [43,44,45].
To provide inhibition of the surface hydration expansion, inhibitors must be adsorbed on the clay mineral surface. Therefore, it is important to understand the mechanism of adsorption and ion exchange of cations and inhibitors on different surfaces. In the following sections, the authors review the adsorption of different cations and inhibitors on external basal surfaces, external clay edges, and interlayer basal surfaces of the clay minerals. The MD simulations studies on adsorption properties started in 2003, while the studies which focus on swelling started much earlier.
2.3.1. Adsorption on the interlayer basal surfaces
In the process of hydration of exchangeable cations in the interlayers, the water molecules penetrate the stacked layers, resulting in an increase of the interlayer space. The research of the organic molecules’ ability to inhibit the surface hydration expansion of clay minerals should be focused on studying the mechanism of intercalation and the swelling processes. The interfacial structure and interactions of the clay minerals with organic compounds at the atomic scale and the force that drives the organic compounds to enter the clay interlayers are still unclear.
The computer molecular modeling method has contributed to the understanding of interaction, distribution, and configuration of the alkyl chains of organic cations in the interlayer space at the molecular scale. Many studies have been focused on the adsorption of organic cations on the internal surfaces of clay minerals by molecular simulation.
Zhang et al. [46] applied the classical all-atom molecular dynamics simulation to evaluate the interfacial interactions between kaolinite interlayer surfaces and DMSO and the force driving the DMSO molecules to enter the interlayer space. The simulation model is presented in Fig. 8. The results show that penetration of the DMSO molecules into the kaolinite interlayer is stimulated by attraction forces of the kaolinite octahedral and tetrahedral surfaces. The hydroxyl groups on the octahedral surface are acting as H-donors attracting the S=O groups of the DMSO molecule forming the hydrogen bonding interaction. The tetrahedral surface demonstrates hydrophobic ability and attracts the methyl groups of the DMSO molecule forming a hydrophobic interaction. The results could be useful for the experimental design of kaolinite intercalation and the related applications.
Zhang et al. [47] developed a series of kaolinite-NMF (N-methylformamide) complex models with various numbers of NMF molecules in the interlayer space and studied the progressive stage of NMF intercalation in kaolinite. In the process of interfacial interaction in the kaolinite-NMF system, both the octahedral and the tetrahedral surfaces show a high binding affinity to the NMF molecules, which is the driving force of NMF intercalation in kaolinite. The results are presented in Table 1. This study provides an insight into the basal spacing evolution and the interfacial structure of kaolinite-NMF complexes, which can enhance the understanding of kaolinite intercalated by small molecules.
Wu et al. [48] applied the molecular dynamics simulation to examine the interlayer microstructures of montmorillonite intercalated with CnmimCl with different chain lengths and studied the intercalation amount, arrangement, and energy. The results show, that with an increase in the chain length of organic cations, both the basal spacing of montmorillonite and the corresponding energy also increase. The results provide insight for understanding of the synthesized Cnmim+-montmorillonite microstructure and the guidelines for further engineering applications.
Willemsen et al. [49] studied the adsorption of phthalate esters on the smectite clay surfaces and performed a detailed analysis of the adsorption free energy. The results show that the phthalate affinity to the clay surface can be attributed to a small contribution of Van der Waals forces and a large favorable entropic contribution.
Yang et al. [50] studied the mechanism of CHPTA (3-chloro-2-hydroxypropyl trimethylammonium chloride) adsorption on the dry and the hydrated montmorillonite surfaces using the DFT method. The interactions between the CHPTA and H2O molecules and Mt are analyzed in terms of adsorption energy, Mulliken bond population, and charge transfer. It was found that CHPTA is mainly adsorbed on the surfaces by hydrogen bonds and electrostatic attraction. In the presence of water molecules, the adsorption of CHPTA is promoted by H2O. A cooperative adsorption effect is provided by enhancing the MMT-CHPTA electrostatic force and forming more hydrogen bonds and H-Cl bonds between the CHPTA and H2O molecules and Mt.
Kristóf et al. [51] applied molecular simulation to model, evaluate, and compare the incorporation ability of the formamide, urea, and N-methylformamide molecules in the kaolinite interlayer space. The authors evaluated the characteristic basal spacings of the intercalation complexes and compared the orientation and density distribution of the guest molecules and their atomic pair correlation functions. They also calculated typical intermolecular interaction energies and estimated the relative mobility of different substances.
Borrego-Sánchez et al. [52] studied the adsorption characteristics of a surfactant (tallow amine ethoxylate) in the interlayer space of montmorillonite by experimental and computational methods. The results show that the surfactant is adsorbed in the montmorillonite interlayer space forming hydrogen bonds between the H atoms of the surfactant and the basal tetrahedral O atoms on the interlayer surface. The resulting hydrogen bonds and electrostatic interactions between the cations and the phyllosilicate surface are the main driving forces of the adsorption.
However, in spite of many published studies on the mechanism of adsorption, there is still a need for an in-depth and systematic investigation of the adsorption of effective hydration inhibitors on the montmorillonite surfaces at the molecular level.
2.3.2. Adsorption on the external basal surfaces
As stated above, the structures of clay minerals and cations are responsible for the adsorption properties of these cations on clay minerals. Teich-McGoldrick et al. [53] investigated the adsorption mechanism of UO22+ on the basal surfaces of muscovite and found that the existence of other ions (K+) has an imbibition effect on the adsorption of UO22+ cations.
Greathouse et al. [43] also used the MD simulation method to investigate the effect of carbonate and sodium ions on the adsorption of uranyl ions on the external basal surfaces in clay minerals, including pyrophyllite, beidellite, and Mt. They found that the uranyl ions in the aqueous carbonate complexes are less likely to interact with negatively-charged clay minerals. They also showed that the presence of Na+ ions has little impact on uranyl adsorption.
Considering the impact of different cations, Underwood et al. [54] studied the adsorption of different cations on montmorillonite basal surfaces and showed that the cation adsorption varies in the following order: K+ > Na+ > Ca2+ > Cs+ > Ba2+. Similarly, Kalinichev et al. [55] studied a number of monovalent (Li+ , Na+ , K+ , Rb+ , Cs+) and divalent (Mg2+ , Ca2+ , Sr2+ , Ba2+ , Ni2+ , UO22+) cations to quantify the adsorption and transport mechanism of hydrated cations on the basal surfaces in different clay minerals, including illite, smectite, and interstratified illite/smectite clays.
Although the adsorption capacities of various cations have been investigated and compared, there are relatively few studies on the effect of other coexisting ions, such as Na+, K+, and Ca2+, on the adsorption process. Most of the MD simulation studies are conducted at room temperature and normal pressure. Further studies should be focused on suppressing the surface hydration expansion of clay minerals under the conditions of shale gas extraction.
2.3.3. Adsorption on the external clay edge surfaces
In the previously published studies on the surface properties of water/clay mineral systems, the MD simulation method has been focused almost exclusively on the basal surfaces and interlayer nanopores. Although the edges take up only a small percentage of surface in a clay system (about 1%), the external edges of the clay particles are highly reactive compared with the chemically inert basal planes.
The edge surfaces can react chemically with water and provide sorption sites for cations. In the process of swelling, water and inhibitor molecules migrate from the external surfaces and micro-pores into the interlayer, passing between the clay edges. Therefore, the influence of the clay edge surfaces needs to be investigated.
Though the classical CLAYFF model has not been parameterized to simulate clay edge surfaces, Martins et al. [56] found that the CLAYFF force field could be applied to reliably simulate the edge surfaces in pyrophyllite by the quantum mechanical calculation method. Other MD simulation studies have been performed to study the clay edges in different clay minerals including smectite. The CLAYFF model was originally developed as a robust and flexible force field model for classical molecular simulation. However, with the wide use of the model, its limitations have become evident. One of the most important limitations is the difficulty to model accurately the edges of the finite-size nanoparticles and pores rather than the infinitely-layered periodic structures.
Pouvreau et al. [57] proposed a systematic approach to solving this problem by developing a specific bending parameter in the system metal−O−H (Me−O−H): Ebend = k (θ – θ0)2. The bending energy parameter is used to describe the structure and dynamics of singly-protonated hydroxyl groups on the mineral surfaces, particularly the edge surfaces. A similar parametrization of the Me−O−H bending parameter has been defined for Si and Al atoms in tetrahedral coordination. The introduction of the bending parameter improves the CLAYFF model and provides more accurate and reliable modeling of the clay particle surfaces and edges.
Based on the DFT method, Peng et al. [58] studied the adsorption of CaOH+ on the (001) and (010) surfaces in montmorillonite. It was shown that the CaOH+ cations are more steadily adsorbed on the (010) edge surface than on the (001) surface.
Zhang et al. [59] performed the FPMD simulation to derive the complexation mechanism of heavy metal cations on the clay edges at elevated temperatures. For the (010) edges, regardless of coordination of the Al(III) atoms, the octahedral vacancies provide the most favorable complexing sites due to their significantly higher free energies.
Therefore, further studies should focus on the adsorption mechanism of water molecules and inhibitor molecules on the external clay edges. Instead of applying the empirical force field models, more advanced force field models should be developed and verified.
3. Conclusions
In spite of a big number of MD simulations performed on the processes occurring in the clay minerals, many issues of the underground hydration expansion of clay minerals remain unsolved. In this section, we have summarized the unsolved issues, with the aim of providing some suggestions for future researchers.
1. Heterogeneity of mineral surfaces. In the previous studies, the external basal surfaces of clay minerals are generally considered homogeneous in their properties, e.g., a single value is adopted to represent the diffusion coefficient of hydrated ions for the entire external basal surface. However, due to the heterogeneity of clay mineral surfaces, in the MD simulation studies, the site-specific information about the structure, dynamics, and energetics of interfacial aqueous species should be taken into consideration when dealing with aqueous substances on different individual surface sites of the clay minerals.
2. MD simulation is a force-field-based nanoscale simulation method and the correctness of MD simulations depends on the selected force field model. Although many down-scaling methods (i.e., ab initio MD simulations) and experimental methods have been adopted to provide initial information or to confirm the correctness of MD simulations, many MD simulations are based on empirical force fields. Therefore, establishing an appropriate force field model for MD simulations, for instance, the force field for adsorption on external clay edges, is of great significance.
3. Clay minerals are affected by external forces, including underground pressure and other mechanical perturbation caused by the geological repository. However, these factors are seldom considered. Most previous MD simulations were performed under free swelling conditions, while in reality, the swelling of compacted bentonites occurs under the confining pressure conditions. Therefore, it is necessary to consider the impact of confining pressure on swelling deformation and expansion.
4. Due to the limitation of computational power, MD simulations cannot deal with large spatial scales and long temporal scales. The preformed MD simulations are restricted to small-size systems and timescales. Hence, up-scaling methods, coarse-grained methods, and multi-scale methods should be adopted in future studies to deal with the computational problem.
5. Computer simulations play an important role in modern research methods, yet the accuracy of widely used empirical force fields (FF) and density functional theory (DFT) exchange-correlation functionals is often unsatisfactory, particularly in the adsorption systems dominated by weak interactions.
References
F. Zhang, B. Lee, and M. Mack, “Wellbore stability modeling with a grain-based rock model,” 49th US Rock Mechanics/Geomechanics Symposium, 311-316 (2015).
M. Attia, M. Elsorafy, and S. D’Angelo, “New engineered approach to replace oil-based drilling fluids with highperformance water-based drilling fluids in the Mediterranean Sea,” North Africa Technical Conference and Exhibition, Cairo, Egypt (2010).
L. Yin and S. Guo, “Full-sized pore structure and fractal characteristics of marine-continental transitional shale: a case study in Qinshui Basin, North China,” Acta Geol. Sin., 93(03), 675-691 (2019).
X. Li, Q. Li, S. Yang, and G. Yang, “Swelling of clay minerals: dual characteristics of K+ ions and exploration of critical influencing factors,” Phys. Chem. Chem. Phys., 21(4), 1963-1971 (2019).
G. Lv, Z. Li, W. T. Jiang, P. H. Chang, and L. Liao, “Interlayer configuration of ionic liquids in a Ca-montmorillonite as evidenced by FTIR, TG-DTG, and XRD analyses,” Mater. Chem. Phys., 162, 417-424 (2015).
G. Xie, P. Luo, and M. Deng, “A novel method for determining the degree of clay swelling in clay–polymer–water systems,” Chem. Technol. Fuels Oils, 52(5), 542-549 (2016).
K. Cheng and Z. Heidari, “Combined interpretation of NMR and TGA measurements to quantify the impact of relative humidity on hydration of clay minerals,” Appl. Clay Sci., 143, 362-371 (2017).
S. Villabona-Estupiñán, J. de Almeida Rodrigues, and R. Nascimento, “Understanding the clay-PEG (and hydrophobic derivatives) interactions and their effect on clay hydration and dispersion: a comparative study,” Appl. Clay Sci., 143, 89-100 (2017).
B. Mantisi, “Generation of polycrystalline material at the atomic scale,” Comput. Mater. Sci., 118, 245-250 (2016).
A. R. Leach, Molecular Modelling Principles and Applications, Longman, New York (1996).
V. Marry and B. Rotenberg, “Upscaling strategies for modeling clay-rock properties,” Dev. Clay Sci., 6, 399-417 (2015).
B. F. Ngouana and A. G. Kalinichev, “Structural arrangements of isomorphic substitutions in smectites: molecular simulation of the swelling properties, interlayer structure, and dynamics of hydrated Cs-montmorillonite revisited with new clay models,” J. Phys. Chem., 118, 12758-12773 (2014).
S. P. Altaner, “Smectite group,” in: Encyclopedia of Sediments and Sedimentary Rocks, Springer, Dordrecht (1978).
M. S. Karmous, “Theoretical study of kaolinite structure; energy minimization and crystal properties,” Nanosci. Eng., 1(2), 62-66 (2011).
H. Sakuma and K. Kawamura, “Structure and dynamics of water on Li+, Na+, K+, Cs+, and H3O exchanged muscovite surfaces: a molecular dynamics study,” Geochim. Cosmochim. Acta, 75, 63-81 (2011).
R. M. Pashley and J. N. Israelachvili, “Molecular layering of water in thin films between mica surfaces and its relation to hydration forces,” J. Colloid Interface Sci., 101(2), 511-523 (1984).
Y. W. Hsiao and M. Hedström, “Swelling pressure in systems with Na-montmorillonite and neutral surfaces: a molecular dynamics study,” J. Phys. Chem. C, 121(47), 26414-26423 (2017).
J. A. Greathouse, R. T. Cygan, J. T. Fredrich, G. R. Jerauld, “Molecular dynamics simulation of diffusion and electrical conductivity in montmorillonite interlayers,” J. Phys. Chem. C, 120(3), 1640-1649 (2016).
B. Rotenberg, “Water in clay nanopores,” MRS Bull., 39, 1074-1081 (2014).
B. Louafi and B. Bahar, “Simple evaluation of the influence of an inert additive on the swelling characteristics of clay soil,” MATEC Web Conf., 149, 02075 (2018).
S. L. Teich-McGoldrick, J. A. Greathouse, C. F. Jove-Colon, and R. T. Cygan, “Swelling properties of montmorillonite and beidellite clay minerals from the molecular simulation: comparison of temperature, interlayer cation, and charge location effects,” J. Phys. Chem. C, 119, 20880-20891 (2015).
L. Sun, J. T. Tanskanen, J. T. Hirvi, S. Kasa, T. Schatz, and T. A. Pakkanen, “Molecular dynamics study of montmorillonite crystalline swelling: roles of interlayer cation species and water content,” Chem. Phys., 455, 23-31 (2015).
Y. G. Chen, C. M. Zhu, W. M. Ye, Y. J. Cui, and B. Chen, “Effects of solution concentration and vertical stress on the swelling behavior of compacted GMZ01 bentonite,” Appl. Clay Sci., 124, 11-20 (2016).
L. Sun, C. Y. Ling, L. P. Lavikainen, J. T. Hirvi, S. Kasa, and T. A. Pakkanen, “Influence of layer charge and charge location on the swelling pressure of dioctahedral smectites,” Chem. Phys., 473, 40-45 (2016).
A. Seppälä, E. Puhakka, and M. Olin, “Effect of layer charge on the crystalline swelling of Na+, K+, and Ca2+ montmorillonites: DFT and molecular dynamics studies,” Clay Miner., 51, 197-211 (2016).
N. Loganathan, A. O. Yazaydin, G. M. Bowers, A. G. Kalinichev, and R. J. Kirkpatrick, “Cation and water structure, dynamics, and energetics in smectite clays: a molecular dynamics study of Ca-hectorite,” J. Phys. Chem. C, 120, 12429-12439 (2016).
W. Zhan, H. Yi, S. Song, Y. Zhao, and F. Rao, “Hydrophobic agglomeration behaviors of clay minerals as affected by siloxane structure,” Coll. Surf. A: Physicochem. Eng. Asp., 568, 36-42 (2019).
J. Zhou, E. S. Boek, J. Zhu, X. Lu, M. Sprik, and H. He, “Molecular simulation study of hydrated Na-rectorite,” Langmuir, 31, 2008-2013 (2015).
Y. Zheng, A. Zaoui, and A. Pasteau, “Interactions between clay portions with various contacts and subjected to specific environmental conditions,” Appl. Surf. Sci., 292, 311-318 (2014).
L. Sun, J. T. Tanskanen, S. Hirvi, T. Kasa, T. Schatz, and T. A. Pakkanen, “Molecular dynamics study of montmorillonite crystalline swelling: roles of interlayer cation species and water content,” Chem. Phys., 455, 23-31 (2015).
H. Li, S. Song, X. Dong, F. Min, Y. Zhao, C. Peng, and Y. Nahmad, “Molecular dynamics study of crystalline swelling of montmorillonite as affected by interlayer cation hydration,” JOM, 70, 479-484 (2018).
J. Peng, H. Yi, S. Song, W. Zhan, and Y. Zhao, “Driving force for the swelling of montmorillonite as affected by surface charge and exchangeable cations: a molecular dynamic study,” Res. Phys., 12, 113-117 (2019).
T. Liu, X. F. Tian, Y. Zhao, and T. Zhao, “Swelling of K+, Na+, and Ca2+-montmorillonites and hydration of interlayer cations: a molecular dynamics simulation,” Chin. Phys. B, 19, 109101 (2010).
L. Zhang, X. Lu, X. Liu, J. Zhou, and H. Zhou, “Hydration and mobility of interlayer ions of (NaX, CaY)-montmorillonite: a molecular dynamics study,” J. Phys. Chem. C, 118, 29811-29821 (2014).
P. Na, F. Zhang, and Y. Li, “Molecular dynamics simulation of Na-montmorillonite and Na/Mg-montmorillonite hydrates,” Acta Phys. Chim. Sin., 22, 1137-1142 (2006).
W. Shen, L. Li, H. Zhou, Q. Zhou, M. Chen, and J. Zhu, “Effects of charge density on the hydration of siloxane surface of montmorillonite: A molecular dynamics simulation study,” Appl. Clay Sci., 159, 10-15 (2018).
L. Sun, C. Y. Ling, L. P. Lavikainen, J. T. Hirvi, S. Kasa, and T. A. Pakkanen, “Influence of layer charge and charge location on the swelling pressure of dioctahedral smectites,” Chem. Phys., 473, 40-45 (2016).
X. Liu, X. Lu, R. Wang, and H. Zhou, “Effects of layer-charge distribution on the thermodynamic and microscopic properties of Cs-smectite,” Geochim. Cosmochim. Acta, 72, 1837-1847 (2008).
L. N. Lammers, I. C. Bourg, M. Okumura, K. Kolluri, G. Sposito, and M. Machida, “Molecular dynamics simulations of cesium adsorption on illite nanoparticles,” J. Coll. Interface Sci., 490, 608-620 (2017).
N. Zhang, X. Liu, C. Li, and C. Liu, “Effect of electrolyte concentration on uranium species adsorption: a molecular dynamics study,” Inorg. Chem. Front., 2, 67-74 (2015).
N. Loganathan and A. G. Kalinichev, “Quantifying the mechanisms of site-specific ion exchange at an inhomogeneously charged surface: case of Cs+/K+ on hydrated Muscovite Mica,” J. Phys. Chem. C, 121, 7829-7836 (2017).
N. Loganathan, A. O. Yazaydin, G. M. Bowers, A. G. Kalinichev, and R. J. Kirkpatrick, “Structure, energetics, and dynamics of Cs + and H2O in hectorite: molecular dynamics simulations with an unconstrained substrate surface,” J. Phys. Chem. C, 120, 10298-10310 (2016).
J. A. Greathouse and R. T. Cygan, “Water structure and aqueous uranyl (VI) adsorption equilibria onto external surfaces of beidellite, montmorillonite, and pyrophyllite: results from molecular simulations,” Environ. Sci. Technol., 40, 3865-3871 (2006).
M. M. Akafia, T. J. Reich, and C. M. Koretsky, “Assessing Cd, Co, Cu, Ni, and Pb sorption on montmorillonite using surface complexation models,” Appl. Geochem., 26, S154-S157 (2011).
M. Franus and L. Bandura, “Sorption of heavy metal ions from aqueous solution by glauconite,” Fresenius Environ. Bull., 23, 825-839 (2014).
S. Zhang, Q. Liu, H. Cheng, F. Gao, C. Liu, and B. J. Teppen, “Mechanism responsible for intercalation of dimethyl sulfoxide in kaolinite: molecular dynamics simulations,” Appl. Clay Sci., 151, 46-53 (2018).
S. Zhang, Q. Liu, F. Gao, R. Ma, Z. Wu, and B. J. Teppen, “Interfacial structure and interaction of kaolinite intercalated with N-methylformamide insight from molecular dynamics modeling,” Appl. Clay Sci., 158, 204-210 (2018).
L. Wu, C. Yang, L. Mei, F. Qin, L. Liao, and G. Lv, “Microstructure of different chain length ionic liquids intercalated into montmorillonite: a molecular dynamics study,” Appl. Clay Sci., 99, 266-274 (2014).
J. A. R. Willemsen, S. C. B. Myneni, and I. C. Bourg, “Molecular dynamics simulations of the adsorption of phthalate esters on smectite clay surfaces,” J. Phys. Chem. C, 123(22), 13624-13636, (2019).
Z. Yang, W. Liu, H. Zhang, X. Jiang, and F. Min, “DFT study of the adsorption of 3-chloro-2-hydroxypropyl trimethylammonium chloride on montmorillonite surfaces in solution,” Appl. Surf. Sci., 436, 58-65, (2018).
T. Kristóf, Z. Sarkadi, Z. Ható, and G. Rutkai, “Simulation study of intercalation complexes of kaolinite with simple amides as primary intercalation reagents,” Comput. Mater. Sci., 143, 118-125 (2018).
A. Borrego-Sánchez, E. Gómez-Pantoja, E. Morillo, T. Undabeytia, and C. I. Sainz-Díaz, “Adsorption of the tallow amine ethoxylate surfactant ethomeen T/15 on montmorillonite,” Appl. Clay Sci., 161, 533-543 (2018).
S. L. Teich-McGoldrick, J. A. Greathouse, and R. T. Cygan, “Molecular dynamics simulations of uranyl adsorption and structure on the basal surface of muscovite,” Mol. Simul., 40, 610-617 (2014).
T. Underwood, V. Erastova, and H. C. Greenwell, “Ion adsorption at clay-mineral surfaces: the Hofmeister series for hydrated smectite minerals,” Clay. Clay Miner., 64, 472-487 (2016).
A. G. Kalinichev, N. Loganathan, B. F. N. Wakou, and Z. Chen, “Interaction of ions with hydrated clay surfaces: computational molecular modeling for nuclear waste disposal applications,” Proc. Earth Planet. Sci., 17, 566-569 (2017).
D. M. Martins, M. Molinari, M. A. Gonçalves, J. P. Mirão, and S. C. Parker, “Toward modeling clay mineral nanoparticles: the edge surfaces of pyrophyllite and their interaction with water,” J. Phys. Chem. C, 118, 27308-27317 (2014).
M. Pouvreau, J. A. Greathouse, R. T. Cygan, and A. G. Kalinichev, “Structure of hydrated gibbsite and brucite edge surfaces: DFT results and further development of the ClayFF classical force field with metal–O–H angle bending terms,” J. Phys. Chem. C, 121(27), 14757-14771 (2017).
C. Peng, F. Min, L. Liu, and J. Chen, “The adsorption of CaOH+ on (001) basal and (010) edge surface of Na-montmorillonite: a DFT study,” Surf. Interface Anal., 49(4), 267-277 (2016).
C. Zhang, X. Liu, X. Lu and M. He, “Complexation of heavy metal cations on clay edges at elevated temperatures,” Chem. Geol., 479, 36-46 (2018).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.51974270) “Research on matching technology to reduce the complicated conditions and downhole accidents of long horizontal sections” (No.2020CX040201). The authors also thank the authors of all the literature cited in this paper and the researchers who have devoted themselves to addressing molecular dynamic simulation in hydration expansion of clay minerals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 1, pp. 89–98, January – February, 2022.
Rights and permissions
About this article
Cite this article
Pingquan, W., Tao, T., Junlin, S. et al. Review of Application of Molecular Simulation in Inhibiting Surface Hydration Expansion of Clay Minerals. Chem Technol Fuels Oils 58, 63–76 (2022). https://doi.org/10.1007/s10553-022-01352-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-022-01352-0