The present work focused on increasing the yield, energy efficiency, and environmental friendliness of the existing (conventional) method for preparing fire-resistant fluids (FRFs). A series of fire-resistant phosphate esters were synthesized. The reaction occurred vigorously under much milder conditions even without a catalyst if anhydrous alcoholates/phenolates dispersed in an excess of an aromatic hydrocarbon solvent were used. The proposed synthetic method increased the yield of target product by —10% as compared with the conventional one. In addition, the phosphate esters obtained by this method were practically free of tars even before vacuum distillation and had acceptable color and optical density. The proposed technology for synthesizing phosphate esters could produce FRFs with the physicochemical and operating properties required to operate over broad ranges depending on the product application by varying the quantitative and qualitative compositions of the alcohols and/or alkylphenols. In particular, a mixed friary, phosphate with properties meeting basic regulatory requirements for use in power-plant turbine control and lubricating systems was produced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Fire-resistant fluids (FRFs) based on alkyl/alkylaryllaryl phosphate esters are widely used in the coal, metallurgy, energy, engineering, and other industrial sectors because of their extremely high self-ignition temperatures (up to 750°C for aryl phosphates). For example, they are used in coal-mining machine hydraulic systems, pressure casting hydraulic systems, control and lubricating systems of power-plant steam turbines [1], and other technological equipment with elevated fire hazards.
Other compound classes with the required fire resistance are known and include aqueous mixtures of polyglycols (and their esters) and polysiloxanes. However, several operating properties such as gel point, lubricating performance, heat resistance, and corrosion aggressiveness of the organic phosphates were superior to those of the aforementioned analogs so that they were less preferred [2]. Currently, the demand of the Russian Federation for the aforementioned FRFs is supplied by imports, which is responsible for the dependence of several industrial sectors on foreign manufacturers. Thus, the importance of developing economically and technologically effective synthetic methods for phosphate esters can hardly be overestimated.
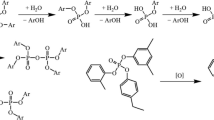
The production technology for phosphate esters has essentially not changed since their industrial synthesis began already in the 1960s [3]. The process is energy intensive and lengthy (12-14 h), is carried out at 140-150°C, and releases as many moles of corrosive gaseous HC1 as the total alcohols/phenols [4]. Many catalysts for phosphorylation of hydrocarbon hydroxyls are known. However, they are all Lewis acids, usually Group II, III, and IV metal halides. Thus, the process can be written briefly using the synthesis of fire-resistant turbine oils from alkylphenols as an example as follows:

The crude product was worked up with base and vacuum fractionated to remove unreacted phenols and partial esters.
It is noteworthy that work on eliminating the aforementioned problems is being conducted. For example, esterification of POC13 by a mixture of a synthetic alkylphenol and unsubstituted phenol under interphase transfer conditions during contact of the organic and alkaline aqueous phases in two steps wherein the reaction mixture was successively heated to 50-60°C and cooled to 20-25°C was proposed [5].

The crude product was worked up with base and vacuum fractionated to remove unreacted phenols and partial esters.
It is noteworthy that work on eliminating the aforementioned problems is being conducted. For example, esterification of POC13 by a mixture of a synthetic alkylphenol and unsubstituted phenol under interphase transfer conditions during contact of the organic and alkaline aqueous phases in two steps wherein the reaction mixture was successively heated to 50-60°C and cooled to 20-25°C was proposed [5].
The main advantages of this method for preparing FRFs are:
lower energy consumption because the reaction occurs at much lower temperatures and without a catalyst because alkali-metal alkylphenolates are significantly more O-nucleophilic than alkylphenols.
separation of NaCl as a side product instead of HC1, which improves the environmental friendliness of the process and the service life of the equipment.
A drawback of this method is the relatively low yield of target product. This was explained first by the incomplete conversion of the starting phenols into their sodium salts because of the lack of factors shifting the equilibrium to the right and, second, by hydrolytic side reaction of the phosphorylating agent with the large amounts of H2O and reacted alkali in the reaction mixture. In this instance, the problem could be solved by using interphase transfer catalysts such as quaternary ammonium salts (e.g.. Aliquat 336) [6].
We selected another route, i.e., the process was carried out in an excess of an aromatic hydrocarbon solvent with complete azeotropic (H2O — xylene) removal of 1120 from the reaction mixture [7]. This avoided undesired reactions such as partial or complete hydrolysis because the only nucleophile in the system was the alkyl phenolate.
Figure 1 shows the target-product yield, i.e., the complete phosphate ester, as a function of the degree of dehydration of the reaction mixture, which was determined as the percent of actually collected distillate vs. the theoretical amount calculated from Reaction (2).
An additional advantage of working in anhydrous solution is that the resulting NaC1 can be filtered rather than rinsing the crude product. This makes the process more environmentally friendly and convenient from a technological viewpoint.
The phosphorylation temperature was reduced to ambient (20-25°C) because the synthesis kinetics of triaryl phosphates from phenoxide anion and POC13 at positive process temperatures was shown to occur practically instantaneously so that the reaction rate depended little on the reactor temperature (except for cases where the phenyl ring has an electron-accepting group) [8]. The rate was limited mainly by the rate of addition of POC13. The previous results [8] led to the conclusion that the key reaction could be carried out in the shortest time possible determined only by the ability to remove heat from the reaction mixture because the process was exothermic (~35 k7/kg for triphenyl phosphate). Phosphorylation at room temperature could avoid side polymerization reactions and produced a product of acceptable appearance even before vacuum distillation.
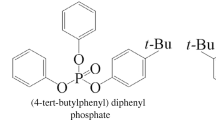
Thus phosphorylation of mixtures of synthetic alkali-metal alkylphenolates of various structures as anhydrous suspensions in an aromatic hydrocarbon can produce at room temperature in almost quantitative yield (>95%) a target product with physicochemical and operating properties applicable to fire-resistant oils based on triaryl phosphates.
The proposed method could produce FRFs based on phosphate esters with the required physicochemical properties, i.e., one parameter or another (viscosity, density, gel point, etc.) could be varied over broad ranges by varying the quantitative and qualitative compositions of the alcohols and/or alkylphenols. A turbine-oil sample complying with the main regulations of RD E0 1.1.2.05.0444-2016. Table 1 presents the characteristics of the obtained product and the requirements for this type of compounds.
References
L. D. Quin, Guide to Organophosphorus Chemistry, John Wiley & Sons Inc., New York, 2000.
K. I. Ivanov (ed.), Fire-resistant Turbine Oils [in Russian], Khimiya, Moscow, 1974, pp. 14-30.
USSR Pat. Appl. No. 254524.
A. G Vainshtein, M. M. Razarenova, et al., RUPat. 2,081,877, Oct. 10, 1996.
A. G Vainshtein, A. E. Shipov, et al., RU Pat. 2,165,427, Apr. 20,2001.
S. Asi, H. Nakamura, M. Tanabe, et al., Ind. Eng. Chem. Res., 33, No. 7, 1687-1691 (1994).
A. S. Medzhibovskii, A. V. Dementev, et al, RU Pat. 2,667,059, Sept. 14, 2018.
C. F. P. Machade e Silva and F. Cajaiba da Silva, Org. Process Res. Dev., 6, No. 6, 829-832 (2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Mosel, No. 5, pp. 35 — 37, September — October, 2019.
Rights and permissions
About this article
Cite this article
Medzkibovskii, A.S., Kolokol’nikov, A.S. & Savchenko, A.O. Fire-Resistant Fluids Based on Organic Phosphates Prepared by Low-Temperature Phosphorylation. Chem Technol Fuels Oils 55, 557–560 (2019). https://doi.org/10.1007/s10553-019-01066-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-019-01066-w