A study was carried out on the effect of thickeners on the physicochemical properties of low-temperature greases. The dispersed phases were the lithium soap of stearic and 12-hydroxystearic acids, lithium soap of 12-hydroxystearic acid and 2-ethylhexyl borate, complex lithium soap of 12-hydroxystearic acid and azelaic acid, complex lithium soap of 12-hydroxystearic, sebacic, and boric acids, diurea, Aerosil, a polymer thickener and its hybrids with Aerosil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The content of the dispersed phase (thickener) in greases is usually from 10 to 20%. However, despite this relatively low concentration, it is precisely the thickener that determines the major operational characteristics of greases. The dispersed phase of greases [1, 2] most often consists of salts of fatty acids (simple, complex, and mixed soaps), solid hydrocarbons such as ceresin, paraffin, and petrolatum, inorganic thickeners such as organobentonite, silica gel, graphite, and carbon black, and organic thickeners such as polyurea, pigments, and polymers.
The thickener has an effect on the antifriction and protective properties, water resistance, colloidal stability, mechanical and antioxidant stability of greases. In tarn, the operating conditions, namely, load, temperature, velocity, and environment, dictate the selection of a particular thickener for greases [3, 4].
Soaps of high-molecular-weight carboxylic acids or soaps of the same acids but obtained upon the saponification of various natural fats and synthetic fat substitutes are used as the dispersed phase to obtain ordinary soap greases. Boner [1] has established that the introduction of low-molecular-weight acids into soaps can significantly alter the properties of derived greases. Thus, it is possible to increase the dropping point and improve the tribological, viscositytemperature, and other properties of soap greases.
Greases with a dispersed phase containing not only soaps but also salts of low-molecular-weight acids are called complex greases. This group includes greases with a thickener containing soaps of the same cation of high- and low-molecular-weight (water-soluble) acids. Such complex greases have found common use. The most commonly used of these are complex lithium (cLi) and complex calcium (cCa) greases [5].
Complex calcium greases are commonly used in heavy load junctions and friction junctions operating at high temperature since these greases have a high dropping point (over 200°C) and good tribological properties [6]. The advantages of complex calcium soap include high thickening capacity, insolubility in water, good heat resistance, low susceptibility to syneresis, and low cost. However, complex calcium greases have serious disadvantages such as hardening at high temperature and upon exposure to moisture and hygroscopicity.
Complex lithium soaps are complex compounds of the lithium soap of a high-molecular-weight fatty acid and lithium salts of low-molecular-weight organic and inorganic acids [7]. The advantages of complex lithium soaps include high mechanical, colloidal, and heat stability, good water resistance, and excellent tribological properties. The major saponifiable component of cLi greases is 12-hydroxystearic acid. The complexing agents are dihydric carboxylic acids such as adipic, azelaic, sebacic, and terephthalic acids as well as inorganic boric and phosphoric acids [8, 9]. Fibers of the dispersed phase of complex soap, in comparison with simple lithium soaps, have a more branched structure, which accounts for the less pronounced temperature dependence of the rheological properties of cLi greases as well as better tribological properties [10]. However, complex lithium greases have disadvantages such as low thickening capacity in comparison with ordinary lithium soaps, which leads to the need for increasing the amount of thickener in the grease and reducing the efficiency of functional additives. Furthermore, the manufacture of cLi greases requires greater energy expenditures and a more complex industrial process, which along with the increased content of the dispersed phase components, makes these greases more expensive to produce [6, 11].
Greases containing poly-urea as the dispersed phase are among the lubricating materials meeting strict modem requirements. Such thickeners are obtained by the reaction of polyisocyanates with arylamines [12]:
Greases made using from polyurea derivatives have a number of advantages [13] including high mechanical and antioxidant stability, good injectability, excellent adhesion to various materials, prolonged durability. Polyurea greases have a broad range of operating temperatures from -70°C to 200°C [13]. The upper working temperature limit for oligourea greases is a function not so much of the stability of the thickener, whose decomposition begins at about 250°C, as of the heat resistance of the base oil [14, 20]. The reason for the prolonged durability of polyurea greases in a broad temperature range may be the capacity of polyurea fibers to thicken with increasing temperature, while the soap base of the grease, as a rule, softens with increasing temperature [15, 16].
Comparative testing of multipurpose lithium greases with polyurea greases showed that the polyurea greases are superior to lithium greases in their antiscuff and wearresistance properties, dropping point, water resistance, usefulness at high temperature, and injectability [17]. The durability of poly-urea greases is attributed to their lack of ash and the presence of functional amine groups, which inhibit oxidation. The metal cation in the thickener of soap greases may catalyze oxidation and thereby reduce the working life of the grease at high temperatures [18, 19]. In light of these advantages as well as good adhesion, hydrolytic and chemical stability in contact with aggressive media and steam, polyurea greases are shown effective in machine building, metallurgy, and steelmaking [20]. A serious disadvantage of polyurea greases is the high toxicity of the starting raw material components, in particular, polyisocyanates and aromatic amines.
There has recently been considerable interest in greases made with crystalline nonpolar polymers. Upon thermomechanical dispersion in the base oils and subsequent cooling, such polymers are capable of forming structured systems similar to soaps and solid hydrocarbons and act as thickeners at concentrations above 6% [21]. Polypropylene is a nonpolar polymer, which has found greatest use as such a thickener for greases.
The production and use of polymer greases are not very common. In comparison with greases containing a lithium thickener, these greases have a number of advantages such as compatibility with additives, an inert thickener system, enhanced film thickness, as well as resistance to moisture and aggressive chemical compounds [22,23,24]. Polymer thickeners have less affinity to friction elements than soaps and do not compete with additives for access to contact surfaces. This provides for improved function for wear resistance and anti-scuff additives and corrosion inhibitors as well as the feasibility of using less polar additives, which are usually expelled by soap from the contact zone [22,23,24,25].
Polymer greases are preferred for the lubrication of parts made with aluminum, copper, and other various alloys, while excess alkali in the soap system can cause coloration and corrosion. The inert nature of polymer materials provides for compatibility with most greases, which avoids problems in going from one thickener system to another and permits the development of hybrid products. These thickeners are highly suitable for the food industry due to the nontoxicity of polypropylene [22].
In addition to soap and organic thickeners of various types and structure, thickeners derived from inorganic materials, in particular, modified pyrogenic silicon dioxide (aerosil) with high dispersion, hydrophobicity, homogeneous particle size, and large surface area are commonly used. Aerosil particles form a strong structured framework of the grease upon mechanical treatment in the mixture with the dispersion medium, are capable of rapid restoration of bonds after their decomposition, and do not require hightemperature thermomechanical dispersion during preparation of the grease. Due to its properties, pyrogenic silica is commonly used in the manufacture of hightemperature greases as well as greases for use in chemically aggressive media [5].
This communication provides a comparison of the physicochemical and operational characteristics of low-temperature greases with thickeners derived from similar and complex lithium soaps, diurea, silica gel, and nonpolar polymers. These results may be used in the development of multipurpose low-temperature greases (LTG) with given operational characteristics (low- and high-temperature as well as tribological properties).
The samples were prepared in a research reactor with a stirring device equipped with a heating jacket containing a high-temperature heat carrier.
The dispersion medium was a 1:1 mixture of mineral oil for the manufacture of S-9 chemical fibers and VHVI-4 hydrocracked hydroisomerized base oil. The mineral oil was selected for the balance of its low- and high-temperature characteristics. VHVI-4 hydroprocessing base oil was used in order to increase in the upper limit of the operational temperature range of multipurpose LTG by enhancing the viscosity of the dispersion medium, reducing its volatility, and improving its temperaturedependent viscosity characteristics. In light of the rapid development of oil-directed hydrocracking by Russian refineries as well as the advantages of oils obtained by such processes over mineral oils and relatively low cost in comparison with synthetic components, hydroprocessing oils hold the greatest interest as dispersion media in the manufacture of multipurpose durable LTG in a broad temperature range from -50 to 180°C.
The following components were studied as thickeners:
lithium stearate soap,
lithium 12-hydroxystearate soap,
lithium soap derived from 12-hydroxystearic acid and 2-ethylhexyl borate (EBB),
complex lithium soap derived from 12-hydroxystearic, sebacic, and boric acids,
complex lithium soap derived from 12-hydroxystearic and azelaic acids,
diurea thickener derived from polyisocyanate, octadecylamine, and aniline,
pyrogenic silica (Aerosil),
polymer thickener derived from a mixture of polypropylene with molecular mass 200,000 and polypropylene with molecular mass 140,000,
hybrid derived from the polymer thickener and Aerosil.
In the first step, we studied the dependence of the properties of LTG on the composition of the thickener when the thickener concentration was 12 wt. %. This concentration provides for greases with NLGI consistency grade 1-3 for all the thickener types examined with the exception of the polymer thickener and its hybrid.
In the second step, we prepared and analyzed samples of greases using thickeners in different concentrations to meet the requirement of colloidal stability in the range of 18.0±2.0 wt.% characteristic for most LTG. This index is one of the most important physicochemical characteristics of greases determining their oil-retention capacity and avoidance of oil separation upon storage and use [26].
The following standard methods were used to study the physicochemical characteristics of the grease samples: State Standards COST 5346-78, COST 6793-74, COST 7142-74, COST 7143-73, COST 7163-84, COST 9566-74, ASTM D 1264, ASTM D 1742, and ASTM D 1743.
The structure of the greases was determined using a JEOL JIB-4501 scanning electron microscope manufactured in Japan. For preparation of the grease samples for scanning, they were subjected to three-fold washing with 50 portions of isooctane, centrifuged, and dried at room temperature. Then, a small portion of the solid residue (dispersed phase) was placed onto a carbon conducting adhesive band and dried in vacuum using a rotary vane pump for 12 h. The deposition of the current-conducting layer (platinum, —4 nm) was carried out by magnetron spraying. The electron microphotographs of the samples were obtained with accelerating voltage 10 kV and detection of secondary electrons in magnification 550-10,000 times.
Thickeners derived from lithium stearate and 12-hydroxystearate as well as Aerosil form the strongest structural framework at 12% concentration (Table 1). The grease derived from lithium 12-hydroxystearate has much poorer colloidal stability, has a greater tendency to separate out oil upon storage, has higher effective viscosity at below-zero temperatures. and displays lower effective viscosity at above-zero temperatures. This behavior may be attributed to the presence of polar OH groups in 12-hydroxystearate molecules leading to the formation of twisted and flat thickener fibers incapable of retaining a sufficient volume of the low-viscosity dispersion medium at above-zero temperatures and requiring greater force to destroy the structural framework at below-zero temperatures.
The grease derived from the soap of lithium 12-hydroxystearate and 2-ethylhexyl borate has physicochemical characteristics similar to the grease with lithium 12-hydroxystearate with the exception of a higher dropping point.
Lithium stearate has the optimal mix of colloidal stability, ultimate strength, and effective viscosity. This feature is well known and has found practical application in the development of a number of Russian low-temperature greases. The most well-known of these greases are TsIATIM-201 and VNII NP-286M (ERA).
With a similar percentage of thickener, greases derived from the complex soap of 12-hydroxystearic, sebacic, and boric acids as well as the complex soap derived from 12-hydroxystearic and azelaic acid have weak structure along with low ultimate strength and colloidal stability, accounting for their better low-temperature characteristics in comparison with the other samples. The low dropping point of these samples indicates incomplete formation of the complex soap due to low concentration of the thickener.
The diurea thickener sample has the lowest ultimate strength (120 Pa) and effective viscosity at 20°C (7 Pa·sec) as well as low colloidal stability and high resistance to oil separation upon storage, which, taking account of the results of Garshin et al. [28], indicate low thickening capacity of diurea of the given composition in the dispersion medium containing VHVI4 hydroprocessing oil.
The LTG sample with Aerosil as the thickener has the best high-temperature properties, good colloidal stability, and low tendency for oil separation upon storage.
The volatility indices are similar for all the samples and a function of the composition the dispersion medium as well as independent of the type of thickener. The higher volatility of the grease sample containing 2-ethylhexyl borate in comparison with the other samples is attributed to the higher volatility of the borate. Hence, the capacity of additives derived from 2-ethylhexyl borate to improve the high-temperature characteristics of lithium greases appears doubtful despite their higher dropping point.
All the samples with the exception of greases with Aerosil as the thickener have similar wear spot diameters. The worse anti-wear characteristics of the silica gel grease in comparison with the other samples are attributed to the abrasiveness of the thickener particles.
The level of the low-temperature characteristics given by the effective viscosity at below-zero temperatures is approximately the same for all the LTG samples, which is attributed to the predominant effect of the dispersion medium on this parameter at a given concentration of the thickeners studied. We should note the rapid increase in the effective viscosity for all the grease samples below -50°C, which is also attributed primarily to the properties of the base oils (the hardening temperature for the S-9 oil used is -50°C).
In the second step, we studied the change in the physicochemical characteristics of the grease samples relative to the type of thickener and colloidal stability at 18±2.0 wt.% (Table 2). Due to the use of lowviscosity base oils for LTG, low colloidal stability is a common characteristic, which places special requirements for strict observance of the technical parameters in each manufacturing step.
Retention of the oil within the structural framework of the grease is achieved by the combined action of capillary, adsorption, and chemisorption forces as well as penetration of the oil molecules into the structural elements of the thickener framework. Thus, the colloidal stability of greases depends not only on the viscosity and group composition of the base oil but also the extent of order of the threedimensional framework of the dispersed phase, which is a function of the size and shape of its structural elements as well as the strength of the bonds between them [26].
Thickeners derived from lithium stearate and Aerosil provides for the necessary colloidal stability of the LTG at a concentration of 12 wt.% used in the first step of the study.
An increase in the content of lithium 12hydroxystearate by 1 wt. % led to a significant decrease in penetration along with increased colloidal stability and ultimate strength. In comparison with the grease containing lithium stearate, the higher ultimate strength and effective viscosity at below-zero temperatures as well as the better resistance to being washed out by water correspond to the required level of colloidal stability; the other indices are on the same level.
Similar to the results obtained in the testing in step 1, the grease with lithium 12-hydroxystearate modified by EHB has characteristics close to the values for the sample with only lithium 12-hydroxystearate with the exception of a higher dropping point and greater volatility as well as less resistance to being washed out by water.
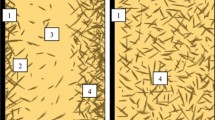
In order to achieve the required level of colloidal stability, the amount of thickener in the complex lithium greases was increased by 21% (for the complex with boric and sebacic acids) and 20% (for the complex with azelaic acid). With these amounts of thickener, samples were obtained with a dense strong structure and high dropping point. In comparison with simple lithium greases, the complex samples with comparable colloidal stability show less resistance to separation of the oil upon storage and greater effective viscosity, which are attributed to features of the fiber structure of the complex lithium thickener (Fig. 1) reflected in increased length and branching in comparison with the fibers of the simple lithium soap.
Increasing the concentration of the diurea thickener to 14% led to a decrease in the penetration but had virtually no effect the ultimate strength of the grease, which value was the least among all samples with soap thickeners and Aerosil. The grease derived from diurea with a similar level of stability as the other samples displays a high tendency for oil separation upon storage and low moisture resistance. This is further evidence for an antagonism between this ureate thickener and the hydroprocessing component of the base oil.
Microphotographs of the Aerosil thickener particles shown in Fig. 1 indicate that the three-dimensional framework of the silica gel greases consists of highly dispersed same-sized nanostructured particles held together by polar interactions. The lower resistance to washing out of the greases with Aerosil thickener than for lithium greases is due to interaction of polar water molecules with hydrophilic Aerosil particles, which form the bulk structure.
Grease samples with polymer and its hybrids with Aerosil have high penetration. Increased Aerosil concentration in the hybrid thickener leads to a slight drop in this index, while the effect of the Aerosil concentration on the ultimate strength and effective viscosity is more pronounced. Grease samples with a nonpolar polymer thickener and its hybrids have a low dropping point related to the melting point of the polymer. The relatively high volatility is attributed to partial decomposition of the high-molecular-weight polypropylene, which occurs upon thermomechanical dispersion during preparation of the grease with the loss of low-boiling components.
The structure of the polymer thickener is represented by spherical particles with 20-60 μm diameter (Fig. 2), which is in accord with the results of our previous study [27]. The high tendency for oil separation and low resistance to wash out by water of the greases with a nonpolar thickener is attributed to the size of its particles and weakness of the interaction between these particles and the dispersion medium. We should especially note the poorest anti-wear properties found for the samples with a nonpolar polymer thickener.
The introduction of pyrogenic silica gel nanoparticles into the polymer thickener leads to their homogeneous distribution over the surface of the polymer particles (see Fig. 2) but has hardly any effect on the structure, shape, and anti-wear properties of the polymer particles. Increasing the Aerosil content in the thickener leads to an increase in the thickness and density of the thickener layer on the polymer surface, reduced oil separation upon storage, and increased moisture resistance.
A comparative analysis of the physicochemical and technical characteristics of LTG samples derived from mineral and hydroprocessing oils with simple and complex lithium soaps, diurea, Aerosil, and a nonpolar polymer as thickeners indicated that
lithium stearate can be recommended as a thickener for LTG used at from -50 to +120°C in lowload friction junctions and at from -60 to +120°C in mediumload friction junctions,
LTG with lithium 12-hydroxystearate soap, including samples with added EHB, as the thickener have higher ultimate strength and low-temperature viscosity than for LTG with lithium stearate along with better resistance to washout by water. These LTG can be used in medium-load friction junctions at from -50 to +120°C under enhanced moisture conditions,
the use of thickeners made with complex lithium soaps give LTG with the highest values of ultimate strength and dropping point with good resistance to washout by water, which permit recommendation for heavy-load friction junctions at from -50 to +150°C,
the use of the diurea thickener in the LTG containing VHVI4 hydroprocessing oil as a component of the dispersion medium is not recommended in light of the low ultimate strength, high tendency for oil separation upon storage, and low moisture resistance,
the Aerosil thickener may be recommended for the development of LTG for long-term use at from -50 to +150°C due to the low temperature for its preparation and its capacity to inhibit oxidation of the dispersion medium upon storage and use. However, we should take account of the insufficient resistance of silica gel greases to washout by water and the need to use additives to improve the tribological characteristics,
nonpolar polymers can be used as the dispersed phase of LTG requiring inclusion of anti-wear and/or anti-scuff additives as well as fillers. However, further work is required to determine the range of application of these materials and the temperature limits for its use requires further study.
References
C. J. Boner, Manufacture and Application of Lubricating Greases, Reinhold, New York (1954).
V. V. Sinitsyn, Assortment and Application of Greases [in Russian], Khimiya, Moscow (1974).
I. G Anisimov, K. M. Badyshtova, et al. in V. M. Shkol’nikov (editor), Fuels, Lubricating Materials, Technical Liquids [in Russian], Tekhniform, Moscow (1999).
S. I. Krakhmalev, Fundamentals of the Efficient Application and Reliability of Technology [in Russian], Ofort, Samara (2010).
Yu. L. Ishchuk, Composition, Structure and Properties of Greases, Kiev (1996).
D. Klamani, Greases and Related Products. Synthesis. Properties. Applications. International Standards [Russian translation], Moscow (1988).
I. S. Povkh, Fact of Technical Factors on the Characteristics of Complex Lithium Greases with Improved Low-Temperature Properties. Author's Abstract of Technological Sciences Candidate's Dissertation [in Russia], Moscow (2015).
Yu. L. Ishchuk and A. D. Stakhursky, NLGI Spokesman, No. 4, 21-24 (1995).
W. H. Dresel, Eleventh International Colloquium, vol. 3, (1998), pp. 2261-2264.
H. Kimura and T. Okaniwa, NLGI Spokesman, No. 3,18-24 (1997).
V. B. Bulgak and L. P. Ishchuk, Chemistry and Technology of Fuels and Oils, No. 4, 1415 (1983).
T. Mang, Greases. Manufacture. Applications, Properties: Handbook [Russian translation], Professiya, St. Petersburg (2010).
A. M. Danilov and A. V. Sergeeva, Ureate Greases [in Russian], Institute of Petrochemical Synthesis, Moscow (1982).
H. Li and L. Xie, Journal of Synthetic Lubrication, No. 8, 39-50 (1991).
C. Kernizan and J. Lorimer, White Paper Lubrisense, No. 14, 12-14 (2013).
Zujian Shen, Fei Geng, Xinxin Fan, et al., Industrial Lubrication and Tribology, No. 68, 5 (2016).
Chevron Oil (UK) Ltd., MultiPurpose Polyurea Grease, Tribology International (1976).
A. M. Danilov, Introduction to Chemotology, Tekhnika, Moscow (2003).
P. S. Venkataramani, R G. Srivastava, and S. K. Gupta, Journal of Synthetic Lubrication, 4, No. 3, 229-244 (1987).
H. Li and L. Xie, Journal of Synthetic Lubrication, 8, No. 1, 39-50 (1991).
V. V. Samgina, I. G Fuks, and M. B. Bakaleinikov, Chemistry and Technology of Fuels and Oils, 9, No. 6, 490-491 (1971).
E. Jacobson, Lubrisense White Paper, No. 7 (2007).
R. Beercheck, Lubes ‘n’ Greases EMEA, No. 78, 18-24 (2015).
J. Leckner and R. Westbroek, NLGI Spokesman, No. 81(1), 34-56 (2017).
J. Shu et al., Tribology International, No. 118, 189-195 (2018).
I. G Fuks, Properties, Manufacture and Applications of Greases [in Russian], Gubkin Institute, Moscow (1970).
V. A. Zaichenko, D. S. Kolybel’skii, P. S. Popov, et al., Khimiya i Tekhnologiya Topliv i Mosel, 54, No. 5,7-12 (2018).
M. V. Garshin et al., Petroleum Chemistry, 57, No. 12, 1177-1181 (2017).
This work was carried out with the financial support of the Russian Ministry of Education and Science (Unique Project Indicator RFMEFI57717X0252, contract number 14.577.21.0252).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 5, pp. 22 — 30, September — October, 2019.
Rights and permissions
About this article
Cite this article
Porfir’ev, Y.V., Popov, P.S., Zaichenko, V.A. et al. Effect of Thickeners on Low-Temperature Greases. Chem Technol Fuels Oils 55, 540–551 (2019). https://doi.org/10.1007/s10553-019-01064-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-019-01064-y