Hydrothermal liquefaction (HTL) of microalgae biomass over an aluminum oxide catalyst with an isomerization component in the neutral and sulfated forms was studied. Gas-chromatographic — mass-spectrometric analysis of the products led to conclusions about the effect of catalyst composition on the gasoline-fraction group composition of the processed bio-oil. The contents of isoalkanes and aromatic hydrocarbons increased whereas the concentrations of S-, 0-, and N-containing components decreased in the HTL products if a catalyst was used. The yield of water-soluble side products also decreased. Sulfated catalysts had greater effects than neutral catalysts on the group composition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Many processes for converting biomass into liquid high-energy products designed to be used as fuels have now been proposed. Hydrothermal liquefaction (HTL) or so-called wet pyrolysis is one of the most promising methods. Moist biomass can be processed, in contrast to dry pyrolysis, so that energy losses for the biomass drying step of ordinary pyrolysis are greatly reduced.
The liquid HTL products are known as bio-oil, a complicated mixture of various organic compounds. These products can be fractionated and reprocessed either with traditional oil feedstock or separately from it to produce liquid biofuels for gasoline and diesel engines [1, 2].
Previously, the composition of the bio-oil gasoline fraction was studied in detail by us [3]. The main components of the bio-oil gasoline fraction were aromatic hydrocarbons and normal and cyclic alkanes. This made it similar to straight-run gasoline obtained from recovered oil. The high contents of toluene, ethylbenzene, and styrene (~50%) indicated that the obtained gasoline fraction had a high octane number. On the other hand, this product could not be recommended as a fuel additive without additional refinement such as hydrofining and soft hydrocracking because of the significant amounts of phenols, organic sulfides, and N-containing compounds [4].
A heterogeneous catalyst could be used as one possible method for improving the properties of the HTL products. In particular, the effects of Co/Mo/Al2O3, Pt/Al2O3, and Ni/Al2O3 catalysts on the yield and properties of the obtained bio-oil were studied [5]. The heterogeneous catalysts increased insignificantly the bio-oil yield and increased the heat of combustion of the products by decreasing the 0 content in them. The effects of six different catalysts [Pd/C, Pt/C, Ru/C, Ni/SiO2—Al2O3, CoMo/y-Al2O3 (sulfided), and a zeolite] on the composition of the gaseous HTL products were reported [6]. The S content in the obtained oil was reduced below the detection limit if the Ni catalyst was used.
The present work attempted to improve the properties of microalgae biomass HTL products by using an Al2O3 catalyst with an added isomerization component. This system could increase the iso-hydrocarbon contents in the products, which should then increase the gasoline fraction octane number and improve the low-temperature performance of heavier fractions. Previously, this system was used by us for direct hydrocatalysis of coconut-oil triacylglycerides [7].
Catalyst specimens were prepared as follows. Finely disperse Al2O3 was mixed with the isomerization component, treated with an aqueous solution of poly(vinyl alcohol) and orthophosphoric acid, and thoroughly mixed. The resulting thick mass was pressed through an extruder to produce granules that were dried at 115°C for 12 h and calcined at 500°C for 5 h. The isomerization components were Zr, Ti, and Sn oxides. Sulfated forms of the catalysts were also used in series of experiments and were prepared by soaking calcined catalysts in H2SO4 solution (10%) and drying at 200°C. Catalysts granules were lightly ground in a porcelain mortar immediately before HTL experiments for better mixing with the biomass. The catalyst compositions and preparation processes were disclosed in more detail in patents [8, 9].
Biomass of microalga Chlorella that was cultivated in a photobioreactor using the published method [10] was used as the HTL feedstock. Hydrothermal processing used a reactor-autoclave equipped with a heating jacket. The reactor was charged with a suspension consisting of distilled H2O (500 mL) microalga Chlorella biomass (150 g), and catalyst (7.5 g). The biomass was preliminarily dried at 105°C to constant mass. Then, the reactor was heated to 300°C over ~60 min, held at the maximum temperature for —80 min, and cooled by turning off the heat. The condensed HTL products were separated into bio-oil, aqueous phase, and solids including the catalyst.
The gasoline fraction of the bio-oil was separated by atmospheric distillation. Products from the start of boiling to 220°C were collected.
Gas-chromatography — mass-spectrometry (GC-MS) on a TRACESTM GC Ultra instrument with a DSQ II MSD (Thermo Fisher Scientific, USA) and an Rtx-5MS capillary column in programmed temperature mode was used for the analyses. The collected gasoline fraction was dried by calcined Na2SO4, diluted (10x) with CH2C12, and injected (0.1 μL) into the chromatograph. Peaks with intensities above the mean noise level were identified in mass spectra and from retention times as compared to standards. Each identified peak was assigned to one of seven groups: aromatic hydrocarbons, alkanes, isoalkanes, cycloalkanes, phenols, organic sulfides, and pyridines.
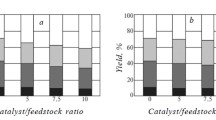
Table 1 presents the yields of bio-oil, gasoline fraction obtained from it, dry solids, and water-soluble substances. The yields of bio-oil and gasoline fraction obtained from it increased with increasing temperature.
The chemical group composition of the bio-oil gasoline fraction was determined using GC-MS. The gasoline fraction was a mixture of many organic compounds. Hydrocarbons of various structures made up a significant fraction.
Also, large amounts of N-, 0-, and S-containing compounds were present. Table 2 lists the quantitative group composition of the bio-oil gasoline fraction.
The results showed that a catalyst had a positive effect on the gasoline-fraction yield. The sulfated catalysts had stronger effects. Also, smaller amounts of water-soluble substances were formed during HTL with a heterogeneous catalyst. This could be due to more complete removal of 0, S, and N atoms from biomass components to form insoluble hydrocarbons.
The change of group composition confirmed that 0, N, and S atoms were more completely removed. In particular, the contents of organic sulfides, phenols, and pyridines decreased in the order: without catalyst > with neutral catalyst > with sulfated catalyst. The contents of aromatic hydrocarbons and isoalkanes increased in the same order. The contents of normal alkanes changed insignificantly.
Catalysts containing an isomerization component of Zr, Ti, or Sn oxide had positive effects on microalgae biomass HTL. In particular, the contents of branched alkanes increased in the gasoline fraction of the obtained bio-oil. This increased its octane number and allowed this product to be recommended as a gasoline additive. Also, a catalyst enhanced removal of 0, S, and N atoms from the biomass components. Further optimization of the process will hopefully obviate the need for additional refining steps of the obtained products.
References
M. S. Vlaskin, N. I. Chernova, S. V. Kiseleva, et al., Therm. Eng., 64, 627-636 (2017).
M. S. Vlaskin, Y. I. Kostyukevich, A. V. Grigorenko, et al., Russ. J. Appl. Chem., 90, 1285-1292 (2017).
M. S. Vlaskin, A. V. Grigorenko, M. S. Kotelev, et al., Khim. Tekhnol. Topt Meisel, 614, 8-11 (2019).
D. L. Barreiro, B. R. Gomez, F. Ronsse, et al., Fuel Process. Technol., 148, 117-127 (2016).
P. Biller, R. Riley, and A. B. Ross, Bioresour Technol, 102, 4841-4848 (2011).
P. Duan and P. E. Savage, Ind. Eng. Chem. Res., 50, 52-61 (2010).
D. P. Mel'nikov, I. A. Antonov, M. S. Kotelev, et al., Chem. Technol. Fuels Oils, 50, 95-98 (2014).
D. P. Mel'nikov, I. A. Tiunov, et al., RU Pat. 2,534,993, Dec. 10, 2014.
M. S. Vlaskin, A. A. Novikov, et al., RU Pat. 2,668,423, Oct. 1, 2018.
M. S. Kotelev, A. A. Novikov, D. S. Afonin, et al., Chem. Technol. Fuels Oils, 48, 8-12 (2012).
Acknowledgments
The work was financially supported by the Ministry of Education and Science of Russia (Unique Project Identified RFMEFI57417X0137).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Mosel, No. 5, pp. 5 — 7, September — October, 2019.
Rights and permissions
About this article
Cite this article
Kotelev, M.S., Kopitsyn, D.S., Vlaskin, M.S. et al. Catalyst Effect on Grout Composition of Microalgae Biomass Hydrothermal Liquefaction Products. Chem Technol Fuels Oils 55, 511–514 (2019). https://doi.org/10.1007/s10553-019-01059-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-019-01059-9