The rheological properties of water-based drilling fluids are heavily influenced by low temperatures and are some of the most important issues for permafrost natural gas hydrate drilling. In this work, we developed a polymer drilling fluid formula and studied its rheological properties at low temperatures. The rheological properties of four different drilling fluids including macro-polymers, amphoteric polymers, sulfonated polymers, and biopolymers were tested. The corresponding rheological-property/temperature response curves were drawn. The response characteristics of the rheological properties with temperature were analyzed. Based on these, a novel research idea was developed to adapt to permafrost drilling a poly-sulfonate drilling fluid system in which sulfonated lignite (SMC) and sulfonated phenolic resin (SMP) were used as the main agents while xanthan gum (XC) served as a flow-pattern modifier. According to orthogonal test results, the optimized drilling fluid formula was base mud + NaCl (20 wt. %) + NaOH (0.1) + SMP (3) + SMC (4) + XC (0.3). Moreover, the rheological-property/temperature response mechanism was analyzed using Fourier transform infrared (FT-IR) spectroscopic tests of the treating agents and scanning electron microscopic (SEM) tests of the mud cakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Natural gas hydrates (NGHs) are formed under phase equilibrium conditions at high pressure and low temperature [1, 2]. Drilling fluids must effectively inhibit decomposition of the hydrates and maintain phase equilibrium during NGH drilling in permafrost areas [3]. However, they should possess good rheological properties for suspending rock cuttings and stabilizing the borehole wall [4]. At present, typical water- and oil-based drilling fluids used for gas-hydrate-bearing sediments have already been researched under permafrost conditions [5]. It was found that inorganic salts and organic antifreezes such as ethylene glycol could inhibit decomposition of NGHs and maintain the borehole stability [6]. Moreover, water-based drilling fluids possess advantages over oil-based fluids for inhibiting gas-hydrate decomposition. Research showed that high pressure had practically no effect on the viscosity of aqueous drilling fluids at low temperatures [7, 8]. It was also established that filter losses of drilling fluids changed little at low temperature.
Low temperatures usually degrade the basic rheological properties of drilling fluids and increase the viscosity and shearing force. Thus, drilling fluids for permafrost should not freeze and should meet applicable requirements for operation at temperatures from 0 to –10°C [9].
The present study represents new work that is fundamentally different from studies of drilling fluids used under conventional conditions and could set the stage for forthcoming NGH exploration in permafrost areas.
Previous work examined a polymer drilling fluid for NGHs in permafrost of the Qilian mountains [10]. Nevertheless, the experiment showed that the drilling fluid [formula: base mud + NaOH (0.1 wt. %) + NaCl (15) + HT (1)] had poor cutting suspension and carrying ability although its viscosity did not change greatly with temperature. Herein, a novel polymer drilling fluid formula with superior rheological properties and low filter losses at low temperature was developed through selection of treating agents and optimization.
Bentonite clay modified by sodium that could be used directly to prepare drilling fluids without Na2CO3, NaOH, NaCl, non-viscosified amphoteric polymer HT-101 (HT), viscosified amphoteric polymer FA-367 (FA), carboxymethylcellulose (CMC), xanthan gum (XC), sulfonated lignite (SMC), and sulfonated phenolic resin (SMP) were used.
A YL-YS-128L digitally controlled laboratory freezer was used to cool drilling fluid samples. The funnel viscosity (FV) (Sioux Chief) was measured using a standard Marsh viscometer. Generally, drilling fluids behaved as Bingham fluids [11] that were described by parameters such as apparent viscosity (AV), yield point (YP), plastic viscosity (PV), and dynamic plastic ratio (ç) that were calculated using a ZNN-D6B rotational viscometer at 300-600 rpm. Other test instruments included a YMS0.01-7.0 electronic density gauge and ZNS-2 API drilling fluid filter tester. Samples of drilling fluid that were cooled to the experimental temperature were immediately removed from the freezer for rheological and filtration tests. Data were measured every 3°C from 9 to –15°C. All tests were performed according to API specifications. FT-IR spectra of treating agents were taken using a 380 FT-IR spectrometer (Nicolet). Mud cake samples formed in API water loss tests were studied using an Inspect F50 scanning electron microscope.
The properties of the base mud affected directly the properties of the low-solid water-based drilling fluid. High-quality Na-modified (5 wt. %) bentonite was used to prepare the base mud, which was evenly mixed and left for 24 h for pre-hydration. The base mud had good low-temperature properties and was responsible for the low solid content of the final drilling fluid used for NGHs, which allowed its freezing point to be depressed and kept its rheological properties stable. At present, halides, formates, and organic alcohols are the three major categories of anti-freezes added to the starting drilling fluid. Studies showed that NaCl was the optimal choice because of its easy use, low cost, good anti-freeze properties, and ability to inhibit NGH decomposition. A NaCl solution (20%) with a freezing point of –15°C was used [9]. The drilling fluid pH was adjusted by adding NaOH (0.1 wt. %) to the system. Thus, the final formula included base mud + NaCl (20 wt. %) + NaOH (0.1). This system could tolerate temperatures as low as –12°C.
Adding NaCl (>3 wt. %) could cause small clay particles to coalesce, which increased sharply the API filtration. Treating agents were required in order to solve this problem.
Salt-resistance additives used in the experiments could be classified into three types, i.e., 1) macro-polymers such as CMC; 2) amphoteric HT and FA polymers; and 3) anti-salt diluents for high temperature and high pressure such as SMC and SMP.
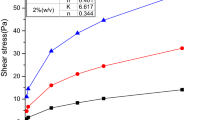
The developed low-solid polymer drilling fluid formula with HT as the main treating agent (1 wt. %) was tested for drilling NGHs in permafrost. Its rheological properties were evaluated. Figure 1 presents the results.
Addition of amphoteric HT copolymer compensated to a large extent for the negative effect of the salt on the drilling fluid. The filtration losses decreased drastically. Figure 1 shows that the FV and PV of the system increased significantly but acceptably as the temperature decreased whereas the YP and dynamic plastic ratio were practically unchanged. The YP of the starting drilling fluid at low temperatures was relatively low, which made it difficult to carry cuttings. The low dynamic plastic ratios reflected the weak shear thinning behavior of the drilling fluid. Although this formula had relatively good viscosity at low temperature, its YP and dynamic plastic ratio did not meet the applicable requirements.
Several other treating agents (concentrations of 1 wt. %) were added to the drilling fluid. Figure 2 shows the results for the temperature dependences of their rheological properties.
Figure 2 shows that the PVs of the macro-polymers, i.e., CMC and FA, increased drastically as the temperature dropped. Their YPs were at high levels, which resulted in rheological failures at low temperatures. Previous studies showed that the temperature had a large effect on the drilling fluid viscosity because the locations of adsorbed water particles in the drilling fluids could change as the temperature changed. The kinetic energy decreased and thermal motion of all particles slowed as the temperature fell so that their movement was hindered. Thus, the viscosity increased [12, 13]. The performance of low-solid polymer drilling fluid could be regulated and controlled by interactions between solid particles and adsorbed groups in the polymer molecular structure. The probability that polymer chains would begin to intertwine with each other and form associated cross-linked structures that could prevent movement of the fluid and bind free water increased as the temperature decreased. In turn, this increased the fluid viscosity and shearing force. The greater the polymer molecular mass was, the greater the viscosity of the drilling fluid was [14]. The above analysis indicated that the use of macro-polymers as treating agents was unreasonable. Conversely, they could act as viscosifiers at low temperatures, which would only degrade the rheological properties of the drilling fluid.
Sulfonated drilling fluids were successfully developed for deep well drilling technology. SMC and SMP were two representative compounds that were used as treating agents with good thermal stability. Figure 2 shows that drilling fluids with separate SMC and SMP additives demonstrated slight viscosity increases at low temperatures. However, their PV and YP were too low and did not meet requirements applicable to drilling fluids designed for NGH drilling in permafrost.
The rheological and filtration properties of the drilling fluid improved to a certain extent as the SMC and SMP concentrations increased (Fig. 3). However, the performance requirements still could not be met.
Adsorption of solid particles and treating agents is known to be strengthened if SMC and SMP are used together. This compensated for the negative effect of the salt and improved the filtration properties [15]. The rheological properties of the formula with base mud + SMP (5 wt. %) + SMC (5) were investigated. Table 1 presents the results. It can be seen that the drilling fluid rheological properties changed little when the components were mixed whereas the filtration losses decreased markedly. Figure 4 shows the change of rheological properties of this system as a function of temperature.
Figure 4 shows that the drilling-fluid viscosity changed little as the temperature decreased. Moreover, the YP and dynamic plastic ratio of this system were small and also changed little with decreasing temperature. Thus, it was concluded that a mixture of SMC and SMP should be used as the main treating agent.
Xanthan gum (XC) is a polysaccharide that is produced via fermentation of glucose or sucrose and is commonly used as a food additive and rheology modifier [16, 17]. A distinctive feature of XC is its high shearing force. XC can significantly increase the dynamic plastic ratio and carrying ability of drilling fluid when used as a treating agent. This stabilizes boreholes. The drilling fluid also had good filtration ability and tolerated well salt and calcium.
The concentration of XC, which acted as a viscosifier, had to be optimized because otherwise the drilling fluid viscosity could increase greatly and degrade the rheological properties. Two systems including base mud with added (0.5 wt. %) mixture of SMC and SMP and amphoteric copolymer HT in the first instance and biopolymer XC, in the second, were studied. Figure 5 shows the results.
The results indicated that XC had better salt resistance and filtration properties than HT. Besides, the PV of the drilling fluid with added XC increased acceptably and less drastically than for added HT. The plastic ratio of the system with copolymer HT was too low to provide normal flow. The YP of the system was also small, which had a negative effect on the ability to carry cuttings. However, these parameters for the system with added XC were high and satisfied the requirements [18]. Thus, XC (0.5 wt. %) was selected as the modifier to improve the low-temperature rheological properties of the drilling fluid.
The optimum concentrations of all reagents had to be determined in order to obtain the final formula for drilling fluid for NGHs in permafrost areas. Orthogonal tests were used for multi-factor and multi-level analyses. Representative tests were selected from the full set of their possible combinations [19]. In this study, there were three factors (three selected additives SMP, SMC, and XC) and three levels (three different concentrations for each additive). The SMP and SMC concentrations were selected from the series 3, 4, and 5 wt. %; XC concentration, 0.2, 0.3, and 0.4 wt. %. The experimental temperature was –12°C.
It was found that drilling fluids of all different combinations possessed low filtration losses that could satisfy the requirements. Because this study was focused on finding systems with good rheological properties, the optimum concentrations were determined based on the PV, YP, and dynamic plastic ratio, namely, their values should vary in the range 0.36-0.48 of the appropriate units. Therefore, the optimum formula for drilling fluid designed for NGHs in permafrost areas was base mud + SMP (3 wt. %) + SMC (4) + XC (0.3).
FT-IR spectra of the used polymers were taken in order to determine the functional groups in their structures (Fig. 6).
The spectrum of XC contained a broad absorption band near 3420 cm–1 due to alcohol –OH stretching vibrations. The peak at 2925 cm–1 was attributed to asymmetric and symmetric C–H vibrations of –CH2 or –CH3 groups. The peak at 1620 cm–1 corresponded to C=O vibrations; at 1416, to C–H vibrations in pyruvic acid and acetyl CH3 groups. Bands at 1061 and 606 cm–1 corresponded to C–O stretching and C–H bending vibrations, respectively. The obtained FT-IR spectra agreed with those in the literature [20, 21]. Based on the results, it was concluded that the XC structure was dominated by –OH and –COOH groups.
The FT-IR spectrum of SMP showed a peak at 3441 cm–1 that was ascribed to phenolic –OH stretching vibrations; at 1612, benzene-ring C=C stretching vibrations; at 1479, C–H bending vibrations in CH2 groups. Bands at 1190 and 1045 cm–1 were due to sulfonic acid –SO3 2– stretching vibrations; at 586 and 524, C–S and S–O bending vibrations, respectively. Thus, the SMP structure consisted largely of benzene rings, phenolic hydroxyls, methylenes, sulfomethyl, and sulfonic groups.
Figure 7a shows SEM images of filter cake from base mud containing sodium bentonite (5 wt. %). It can be seen that its surface was relatively smooth. Fine clay particles reminiscent of flakes formed stacked aggregates with many gaps between the layers.
Figure 7b shows SEM images of filter cake from the system base mud + SMP (5 wt. %) + SMC (5 wt. %); Fig. 7c, filter cake of the final optimized drilling fluid. It can be seen in the first instance that the structure was broken with a large number of fractured micro-cracks that could increase the filter-loss volume. Inclusions of rigid crystalline substances were also observed on the cake surface. In the second instance, the cake surface was compact with few cracks and pores, which could be of benefit to the filtration performance of the drilling fluid.
An attempt was made to analyze the mechanism of action of the fluid. Hydroxyl groups in the XC molecular structure acted as adsorption sites by forming H-bonds to O atoms on the clay surface. Osmotic saturation of the XC hydroxyls increased the zeta-potential and electrical double-layer (EDL) thickness on the surface of the clay particles, thereby increasing their resistance to flocculation. The XC molecular chains adsorbed on the surface of clay particles began to coil and intertwine, forming a networked structure that absorbed a certain amount of free water and increased the fluid viscosity.
SMP molecules adsorbed to the surface of clay particles through H-bonds and van-der-Waals forces. Adsorption of SMP to clay particles was enhanced by SMC if a mixture of SMP and SMC was used. The SMC functional groups were mainly sulfonic and sulfomethyl groups, which are ionic hydrophilic groups with good hydration ability that increase the zeta-potential and EDL thickness [23]. SMP molecules consisted largely of methylenes and benzene rings. Rigid benzene rings were connected to each other through bridging methylene groups. Also, sulfomethyl groups in the molecular chains created powerful steric hindrance. Both these phenomena increased significantly the molecular chain rigidity, thereby preventing coiling and bending of SMP molecular chains adsorbed to the clay [24]. Distances among polymer micelles, bentonite particles, and water molecules decreased whereas the internal friction increased as the temperature decreased. This prevented the molecules from moving and led to small and acceptable increases of the viscosity and shearing force.
Rigid molecular chains adsorbed by clay particles formed many rigid columnar supports that increased the brittleness of the drilling fluid mud cake although the strong hydration of the SMP sulfonates decreased the filter loss to a certain extent (Fig. 7b). As a result, sulfonated drilling fluid had poorer filtration performance than that treated with polymer additives. For this reason, a small amount of XC was added to the system. SMP chains were adsorbed more easily by the clay particles because its molecular mass was smaller than that of XC. Therefore, the fluid viscosity and shearing force did not increase drastically. Flexible XC molecular chains could intertwine with adsorbed rigid SMP chains if the appropriate amount of XC was added. However, the drilling fluid viscosity and shearing force also increased within reasonable limits whereas the filter losses decreased. Thus, the drilling fluid was effectively modified by sulfonated polymers and a biopolymer additive that adjusted its rheological and filtration properties, respectively.
References
Q. Wu, G. Jiang, and P. Zhang, Energy Convers. Manage., 51, No. 4, 783-787 (2010).
Y. Song, L. Yang, J. Zhao, et al., Renewable Sustainable Energy Rev., 31, 778-791 (2014).
L. Zhang, G. Jiang, Y. Tu, et al., J. Chin. Univ. Geosci., 17, No. 3, 276-282 (2006).
G. Jiang, F. Ning, L. Zhang, et al., J. Earth Sci., 22, No. 5, 652-657 (2011).
L. Chen, S. Wang, and C. Ye, Procedia Eng., 73, 318-325 (2014).
S. V. Kokelj, D. Riseborough, R. Coutts, et al., Cold Reg. Sci. Technol., 64, No. 1, 46-56 (2010).
M. Amani and M. J. Al-Jubouri, in: International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production, Sept. 11-13, 2012, Perth, Australia, SPE-157219-MS.
M. Amani and M. Al-Jubouri, Energy Sci. Technol., 4, No. 1, 27-33 (2012).
S. Wang, L. Chen, and Y. Zhang, Nat. Gas Ind., 29, No. 6, 59-62 (2009).
Z. Lu, Y. Zhu, Y. Zhang, et al., Cold Reg. Sci. Technol., 66, No. 2, 93-104 (2011).
J. M. Davison, S. Clary, and A. Saasen, in: SPE Annual Technical Conference and Exhibition, Oct. 3-6, 1999, Houston, Texas, SPE-56632-MS.
W. Halliday, D. K. Clapper, and M. Smalling, in: IADC/SPE Drilling Conference, Mar. 3-6, 1998, Dallas, Texas, SPE-39316-MS.
H. Ebeltoft, M. Yousif, and E. Soergaard, in: SPE Annual Technical Conference and Exhibition, Oct. 5-8, 1997, San Antonio, Texas, SPE-38567-MS.
J. Romero and E. Touboul, in: SPE Annual Technical Conference and Exhibition, Sept. 27-30, 1998, New Orleans, Louisiana, SPE-49056-MS.
J. Yan, J. Southwest Pet. Inst., 2, 1-15 (1982).
R. L. Davidson, Handbook of Water-soluble Gums and Resins, McGraw-Hill, New York, London, 1980, 700 pp.
S. Rosalam and R. England, Enzyme Microb. Technol., 39, No. 2, 197-207 (2006).
J. Yan, Drilling Fluid Technology, 1st Ed., China University of Petroleum Press, Shangdong, 2006.
S. Hao, J. Pet. Sci. Eng., 76, No. 3-4, 109-115 (2011).
S. Faria, C. L. de Oliveira Petkowicz, et al., Carbohydr. Polym., 86, 469-476 (2011).
V. Gunasekar, K. R. Reshma, T. Greeshma, et al., Carbohydr. Polym., 102, 669-673 (2014).
N. Saqib, B. S. Tahira, T. Vincent, et al., Fuel Process. Technol., 92, No. 5, 983-991 (2011).
Y. Zhuang, Z. Zhu, H. Chao, et al., J. Appl. Polym. Sci., 55, No. 7, 1063-1067 (1995).
M. Li, Y. Guo, H. He, et al., J. Chin. Univ. Pet., Ed. Nat. Sci., 34, No. 2, 145-149 (2010).
Acknowledgments
The work was supported by the National Natural Science Foundation of China (Grants Nos. 41272331 and 51204027) and the State Key Laboratory of Geo-hazard Prevention and Geo-environment Protection (Grant No. SKLGP2012Z007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 2, pp. 75 – 82, March – April, 2017.
Rights and permissions
About this article
Cite this article
Sheng, W., Chuan, Z., Chaopeng, Y. et al. Rheological Properties of Polymer Drilling Fluid Developed for Permafrost Natural Gas Hydrate Drilling. Chem Technol Fuels Oils 53, 274–285 (2017). https://doi.org/10.1007/s10553-017-0804-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-017-0804-8