Cracking residual fuel oil separated from a mixture of Azerbaijanian crude oils using catalysts based on aluminum plant sludges containing up to 50-70% Fe2O3 was investigated. It is shown that the yield of liquid products, including motor fuels, upon residual fuel oil cracking at 550 °C rises to 80 and 70 wt. %, respectively. Steam gasification of carbonized and partially reduced catalyst allows production of gas with hydrogen content of up to 72.84 vol. %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The main trends in the petroleum industry are associated with deepening oil refining and improving environmental performance of motor fuels, due to the increase in demand for transportation fuels and involvement in the processing of heavy and high-viscosity oil [1, 2]. Changes in the composition of crude oil (increasing amounts of heavy metals, resins, asphaltenes, etc.) resulted in changes in the structure of the refining industry towards increasing the number and capacity of hydrogenation processes and the need to provide them with sufficient amount of hydrogen. In this regard, processing of heavy oil feedstock to increase the yield of motor fuels and increase the amount of resources containing hydrogen gas for these processes is an urgent problem.
Processes for thermal processing of heavy oil feedstock (residual fuel oil, natural bitumen) have been developed that use magnetic microspheres of energy ashes containing up to 84.2 wt. % iron oxides as activators in order to increase the yield of light oil products. It was found that the yield of distillate fractions for thermal cracking of fuel oil at 500 °C increased from 51.5 to 58 wt. % in the presence of 2 wt. % microspheres, and at 450 °C the yield went up from 24.5 to 64.0 wt. % when magnetic fractions content was increased from 2 to 20 wt. % [3–7].
The processes of thermo-oxidative processing of fuel oil and resins with simultaneous production of distillate fractions and a hydrogen-containing gas have also been developed. The yield of light fractions in the processing of tar reached 52-53% by weight. To increase the content of hydrogen in the produced gas, iron oxide catalysts were used which, as they are recovered in the cracking process, generate hydrogen-containing gas during steam gasification of the carbonized and partially reduced catalyst [8–10].
In order to increase the yield of gasoline and diesel fractions with a simultaneous production of hydrogen-containing gas (HCG), waste (sludge) from an aluminum plant (Ganja, Azerbaijan) was used as catalyst for cracking heavy oil residues. This sludge contained in its composition the following oxides: Al2O3, SiO2, Na2O, CaO, Fe2O3, oxides of indium, and others.
It is known that aluminum plant sludge recycling is a serious environmental problem. Various uses of recycled sludge are known, one of them – the recovery of rare metals and their use as catalysts in various organic synthesis processes, in the manufacture of sulfuric acid, etc. [11] Therefore, studies were carried out using sludges from processing aluminum ore (bauxite and alunite) as catalysts in cracking heavy petroleum residues.
As the feedstock, oil extracted from a mixture of low-paraffin Baku oils (Neftyanye Kamni fields, Guneshli, Gryazevaya volcano) was used [12]. Physicochemical properties of the mixture of oils and residual fuel oil are given in Table 1.
Residual fuel oil, the output of which is about 48.6 wt. %, is used as the primary reserve for deepening oil refining. The content of tar and asphaltenes is 13.1 wt. %, and the content of hydrocarbons (oil) is 86.9% by weight.
For cracking residual fuel oil, catalysts based on two samples of aluminum plant sludges with varying content of Fe2O3 were used, the composition of which is given in Table 2.
Catalysts were prepared by mixing the sludge with measured amounts of MgCO3 and K2CO3 solutions followed by drying at 80-120 °C for 8 hours and calcining at 550 °C for 4 hours. In order to introduce iron oxides into the catalyst composition, the sludge was impregnated with a 10% solution of Fe(NO3)·9H2O. In total, 4 types of catalysts were prepared, all in the form of brick-red color extrudates 3.6-3.8 mm in diameter:
I – initial catalyst,
II – init. + Fe2O3 (70%),
III – init. + MgO (28%), K2O (15%);
IV – init. + MgO (28%), K2O (15%), Fe2O3 (25%).
Liquid cracking products were distilled into fractions: IBP-200 °C, 200-350 °C, and >350 °C. Fractional composition of gasoline was determined by distillation on the Engler apparatus in accordance with GOST 2177-99.
The composition of the gas was determined on a Chrome-5 gas chromatograph (TEGNB [triethylene glycol of n-butryric acid] applied to Sferokhrom-1): column length – 6 m, diameter – 2 mm, carrier gas (helium) flow rate – 30 mL/min.
Group hydrocarbon and component composition of gasoline fractions was analyzed on a “Auto System XL” (Perkin Elmer) chromatograph: Zebron ZB-5 capillary column, 100 m long, 250 microns in diameter, active phase – 5% phenyl + 95% dimethylpolysiloxane, carrier gas – helium.
Total sulfur content of the liquid products was determined by X-ray fluorescence according to the ASTM-D4294 method.
X-ray phase analysis of the catalyst samples was performed on a DRON-3M diffractometer (Cu-Kб source radiation).
Residual fuel oil cracking was carried out in two steps on a flow-type laboratory unit with a fixed bed reactor at a 550-600 °C and a weight hourly space velocity of 2.5 h–1. Cracking of the feedstock to form gaseous and liquid products and coke took place in the first step of the process. Material balance of the residual fuel oil cracking process is presented in Table 3.
The composition of the step I gas was characterized in both cases by a large amount of C2-C4 olefins – up to 51-52 wt. %, and thus can be used as a raw material for petrochemistry. Ethylene content of the total ΣC2 was about 26-27 wt. %. About 0.76-0.81 wt. % of carbon monoxide was present within the composition of the gas indicating that iron oxide (Fe2O3) was reduced to FeO and metallic iron under the cracking conditions. X-ray analysis of the catalyst sample II after cracking residual fuel oil and reaction with steam showed that FeO and metallic á-Fe were present (Fig. 1).
In the second step of the process (700 °C), during steam gasification of carbonized and partially reduced catalyst, processes of catalyst oxidation to Fe3O4 (Fe2O3) and coke gasification to form a hydrogen-containing gas took place. As a result of these reactions, the gas produced in the second step was enriched with hydrogen to about 72 vol. %.
Sample I exhibited the highest cracking activity (Table 3). The output of light fractions was 57.7% by weight. The introduction of an additional amount of Fe2O3 impaired cracking activity of the catalyst – the output of light fractions wa reduced to 31.79% (sample II). Yield of catalysate and light fractions decreased to 6.79 and 25.91 wt. %, respectively, with increasing content of Fe2O3 in the catalyst.
The yield of gas at the second step was 6.91 wt. % when the initial sludge was used, and when 70% Fe2O3 were added to the catalyst the yield of gas increased almost 7-fold to 44.06 wt. %. Hydrogen content of the gas also increases to about 72.84 vol. %. Increased hydrogen yield (sample II) was apparently due to increased amount of reduced iron in the catalyst, and therefore the contribution of iron oxidation by steam was increased.
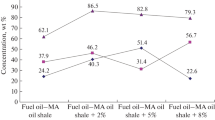
Further studies were conducted using the catalysts III and IV on the basis of oil sludge 2. Residual fuel oil cracking was carried out at 550-600 °C, and gasification of the coked catalyst – at 700 °C. Experimental results are presented in Table 4.
According to the data in Table 4, step I gas contained up to 67-69% of unsaturated C2-C4 hydrocarbons. The yield of liquid products using sample III reached 80% by weight, and of the light fractions – 69.7 wt. % for raw materials, including gasoline and diesel fractions – 26.9 and 42.8 wt. %, respectively. When the catalyst sample IV was used, the yield of light fractions decreased to 55.9 wt. % mainly due to the 200-350 °C fraction, and the yield of step II gas increased slightly.
Gaseous and liquid products of the process contained large amounts of unsaturated hydrocarbons. Liquid cracking products after further hydroforming can be used as components of motor fuels, and the residue above 350 °C (up to 20-25 wt. %) can be returned to the process.
Qualitative characteristics of gasoline fractions obtained by cracking residual fuel oil using catalyst samples III and IV are presented in Table 5. Within the composition of the gasoline fractions in addition to paraffin-naphthene hydrocarbons, cycloolefins, diolefins, and alkenylbenzenes have also been found.
Gasoline fractions contained large amounts of olefin and aromatic hydrocarbons, and thus had high octane ratings (82.3-83.5 by the motor method). Total sulfur content of the gasoline fraction was in the range of 800-850 ppm, representing 23-24% of the original amount in feed. The remaining sulfur was distributed through gases, high-boiling fractions, and accumulated on the catalyst as the silfide FeS.
The data presented in Tables 4 and 5 show that for production of light hydrocarbons and hydrogen-containing gas it is necessary to increase the amount of Fe2O3 in the catalyst and carry out the process under more severe conditions, under which an increased yield of gas is observed due to redox reactions involving the iron oxide component of the catalyst. In the first stage, hydrocarbon feedstock cracking takes place to form gaseous and liquid products and coke deposited on the catalyst surface [10]:
Simultaneously with the cracking of feed, further reduction of the catalyst to FeO (Fe) by coke and gaseous products of cracking takes place.
In the second step the oxidation of the reduced catalyst with steam to produce hydrogen, and gasification of coke deposited on the catalyst surface take place:
Mechanisms presented above show that an increase in the content of Fe2O3 in the catalyst leads to an increase in the content of H2 to about 72.84 vol. % in the step II gas (Table 4).
Based on the yield of distillate fractions (gasoline and diesel), sample III, containing MgO (28%) and K2O (15%), has the highest catalytic activity – up to 70 wt. % (Table 5).
Thus, the process of residual fuel oil cracking by a two-step mechanism has been described here. This process can be used to obtain the components of motor fuel and gas – raw materials for petrochemistry – after the first step, and hydrogen-containing gas with up to 72.84% hydrogen by volume after the second step. Waste (sludge) from the processing of bauxite was used as the catalyst, allowing to produce up to 70% of motor fuels from heavy oil feedstock, as well as solve the ecological problem of sludge recycling.
References
A. V. Borodacheva and M. I. Levinbuk, Rossiiskii Khimicheskii Zhurnal (Russian Chemical Journal), 52, No. 6, 37-43 (2008).
O. B. Braginsky, Oil and Gas Complex in the World [in Russian], publishing house “Oil and Gas” of RGU named after I. M. Gubkin, 640 (2006).
A. K. Golovko and M. A. Kopytov, Izvestiya Tomskogo Politekhnicheskogo Universiteta (Bulletin of the Tomsk Polytechnic University), 315, No. 3, 83-86 (2009).
A. K. Golovko and D. E. Dmitriev, Neftepererabotka i Neftekhimiya (Refining and Petrochemicals), No. 2, 9-14 (2009).
V. I. Sharypov, N. I. Beregovtseva, S. V. Baryshnikov, et al., Khimiya v Interesakh Ustoichivogo Razvitiya (Chemistry for Sustainable Development), No. 3, 287-291 (1977).
E. V. Krivtsov, N. N. Sviridenko, and A. K. Golovko, Izvestiya Tomskogo Politekhnicheskogo Universiteta (Bulletin of the Tomsk Polytechnic University), 323, No. 3, 37-41 (2013).
D. E. Dmitriev, Thermal Conversion of Tar and Heavy Oil Asphaltenes [in Russian], cand. diss. abstract, Tomsk (2010).
M. I. Rustamov, A. D. Guseynova, N. Z. Muradov, et al., Khim. Tekhnol. Topliv Masel, No. 4, 13-15 (1988).
A. D. Guseynova, L. M. Mirzoeva, N. Z. Muradov, et al., Khim. Tekhnol. Topliv Masel, No. 5, 12 (1992).
A. D. Guseynova, L. M. Mirzoeva, R. Yu. Ganbarov, et al., Khim. Tekhnol. Topliv Masel, No. 7, 9-13 (1990).
B. Azizov, Physico-Chemical and Technological Bases of Complex Processing of Liquid and Solid Wastes of Aluminum Production [in Russian], doct. diss. abstract, Dushanbe (2003).
F. I. Samedov, Azerbaijan Petroleum [in Russian], “Elm,” Baku (2011) 412 p.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 5, pp. 25 – 28, September– October, 2016.
Rights and permissions
About this article
Cite this article
Mirzoeva, L.M., Khalafova, I.A. Residual Fuel Oil Cracking Using Alumium Plant Sludges as Catalysts. Chem Technol Fuels Oils 52, 499–505 (2016). https://doi.org/10.1007/s10553-016-0736-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-016-0736-8