We have found chromogenic indicator reactions to test for anti-knock additives to gasolines: ferrocene, cymantrene, and N-methylaniline. Based on these reactions, we have developed methods for fast determination of the indicated additives in automotive gasolines using indicator tubes. The range of analyte contents are: 10–200 mg/dm3 ferrocene, 10–150 mg/dm3 cymantrene, 0.1–1.5 wt.% N-methylaniline.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In 2008, the Technical Regulations concerning Specifications of Automotive and Aviation Gasoline, Diesel and Marine Oil, Jet Engine Fuel, and Furnace Oil were approved, according to which use of metal-containing anti-knock additives (ANA), viz., tetraethyl lead (TEL), bis(h-cyclopentadienyl)iron (ferrocene, FEC), cyclopentadienyl-tricarbomanganese (cymantrene, CMT), and some of their derivatives substituted at the organic fragment, are excluded. The monometylaniline (MMA) content in gasolines is restricted: 1.3 vol. % in class 2 gasoline and 1 vol. % in class 3 and 4 gasolines.
On 31 December 2012, the Technical Regulations concerning Specifications of Automotive and Aviation Gasoline, Diesel and Marine Oil, Jet Engine Fuel, and Furnace Oil (hereafter, Regulations) came into force. In the Regulations, the requirements of metal and MMA content in automotive gasolines were made doubly stringent, and in Belarus use of MMA was even prohibited.
At the same time, even now there are cases of sale at gas stations of gasolines containing metal-bearing ANAs. In view of this, there is an urgent need for an organization for prompt monitoring of ANA content in automotive gasolines. Quick monitoring of fuels at selling places using efficient testing devices will prevent use of automotive gasolines that do not meet the specifications set by the Regulations. The test method for determining MMA is known, which consists in measuring the basicity of the gasoline using bromothymol blue acid-base indicator, which is, however, not specific [1].
The purpose of this work was to create reagent indicator tubes for determining ANA in fuels and development of quick test methods of ANA determination in automotive gasolines at the filling points. We have found new chromogenic indicator reactions on silicon dioxide and based thereupon developed indicator tubes for determining FEC, CMT, and MMA.
For determining FEC, we used a two-stage reaction [2] where potassium ferricyanide (III) (red prussiate of potash, RPP) plays the part of both an oxidant and an indicator: it oxidizes iron (0) to iron (II) and then forms by reacting with the latter water-soluble Berlin blue (SBB) of azure color. The mass spectrum of the reaction products obtained under surface-initiated laser desorption/ionization conditions on an Ultraflex-made Bruker Daltonics mass spectrometer showed SBB ions: K+ (molecular mass M = 39, m/z = 39+) and [FeIIIFeII(CN)6]- (M = 268, m/z = 268-), as well as ions of potassium ferrocyanide (yellow prussiate of potash, YPP): K+ and K3[FeII(CN)6]- (M = 329, m/z = 329). In the reaction mass were also detected cyclopentadienyl (CPD, M = 66, m/z = 66), dicyclopentadienyl (DCPD, M = 152, m/z = 132), and others:

For determining CMT, we used sodium periodate, NaIO4 sorbed on silicon dioxide [3]. The presence in the mass spectra of reaction products with mass numbers typical for manganese oxides and iodate indicates occurrence of reduction of sodium periodate which oxidizes manganese (0) with formation of manganese oxides (III, IV) of dark-brown color and permanganic acid of pink-violet color. The latter decomposes quickly with formation of manganese oxides; in this case, in the test-reaction products we also detected DCPD:

For determining MMA, use was made of reaction of azocoupling (diazotization) of MMA with a strong diazotate, e.g., with 4-methoxybenzoldiazonium-tetrafluoroborate with formation of azo dyes ranging in color from red to dark-brown. According to the mas spectrometric data, the azo dyes formed are 4-methylamino-4’-methoxyazobenzol (I, M = 241, m/z =242) and 2,4-di(4-methoxyphenyl-N-methylaniline (II, M = 386, m/z =387).

MMA also forms with sodium periodate reddish-brown products. The oxidative condensation of MMA occurs with formation of compounds with a quinoneimine structure, which, unlike insoluble manganese oxides, are soluble in acetone. In the mass spectrum of the reaction products (Agilent 5973 chromato-mass spectrometer with a chromatograph of the Agilent 6890 series, USA), the following ions were detected:

where Me denotes – CH3.
The electronic spectrum minimums of the products of test reactions on silicon dioxide matrix are characterized by broad bands, which are responsible for their intense color (Fig. 1). The spectra were recorded on an X-Rite GretagMagbeth ilPro minispectrophotometer (USA).
The characteristics of the indicator tubes: range of determinable contents and color characteristics of the powder – test-reaction product after action of the ANA on the tube, are cited in Table 1.
Preparation of indicator tubes and ANA solutions. 125-mm-long glass tubes with a neck (OOO IMID, Krasnodar) were used. The length of the column containing the filler was 90 mm and its inner diameter, 2 mm.
IT-FEC-Test indicator tubes. High-purity silicon dioxide 4-7 (TU 6-09-4574–81) in 90 wt. part was put in saturated aqueous solution of RPP (analytical grade, Carlo Erba, Italy) in 10 wt. part. The mixture was stirred and dried. The indicator powder obtained was put into the indicator tubes and their mouths were plugged with glass wool.
IT-CMT-Test indicator tubes. High-purity silicon dioxide was put in 100 wt. part in aqueous solution containing 6 wt. part of sodium periodate (99%), the mixture was stirred and dried [3]. The tubes were filled with the indicator powder obtained.
IT-MMA-Test indicator tubes. 13 g of high-purity silicon dioxide was put in 32 ml of aqueous solution of 0.005 M 4-methoxydiazobenzol-tetrafluoroborate and 0.001 M oxalic acid. The powder was then filtered off and dried in air. The tubes were filled with the indicator powder obtained.
Preparation of a series of standard ANA solutions. 20 mg of ANA was dissolved: FES 98% (AKROS, Moscow), or CMT 98% (OOO Toplivnyi Region, Moscow), or 2 g MMA (OAO Volzhskii Orgsintez) in straight-run gasoline (TU 38.001256–90) in a 100 ml measuring flask. The solutions were next diluted with straight-run gasoline to lower concentrations.
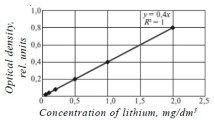
General testing procedure. The method of ANA determination in gasoline using indicator tubes is linearly coloristic. The indicator tube was shortened at the neck and its lower end was immersed in gasoline previously poured into a penicillin vial or a small beaker to a height of 4-5 cm. After the gasoline front rose to the top of the tube, the latter was withdrawn and the length of the colored zone was measured. The ANA content was determined from the calibration curve depicting the dependence of the length of the colored zone of the tube filler on the ANA concentration (Fig. 2 a, b). The relative error of determination in the middle of the calibration curve does not exceed 10% and at the start of the curve at lower concentrations, 25%.
Influence of associated substances on analysis accuracy. In the determination of 60 mg/dm3 of FEC, the presence in gasoline of 40 mg/dm3 of CMT and 1000 mg/dm3 of MMA had no influence on the analysis accuracy. In the presence of larger quantities of MMA, a small brownish green zone appeared in the tubes. In the determination of 60 mg/dm3 of CMT, 90 mg/dm3 of FEC and 1000 mg/dm3 of MMA had no influence on the analysis accuracy. In the determination of 0.1-1 wt. % of MMA, 60 mg/dm3 of FEC and 40 mg/dm3 of CMT had no influence on the analysis accuracy. Thus, only in the determination of CMT did we observe an interfering effect of MMA that forms with IT-CMT-Test a brown product (Fig. 2 b, straight curve 2), which, unlike insoluble manganese oxides, is soluble in acetone.
The CMT and MMA contents in gasoline, where both of them occur simultaneously, were determined as follows. At first the MMA content was determined using IT-MMA-Test in accordance with Fig. 2 b, straight curve 1, and then the length of the colored zone h 1 that corresponds to the segment of the straight curve 2, which is proportional to the found MMA concentration, was determined by extrapolation to the straight curve 2 in Fig.2 b. Thereafter, using IT-CMT-Test we determined the length of the colored zone h 2 that corresponds to the sum of CMT and MMA. The CMT content corresponds to the difference h = h 2 – h 1. If the dried IT-CMT-Test is put in acetone, the azo dye formed in the reaction with MMA rises to the top through the tube, while the manganese compounds do not move.
The automotive gasolines were tested using the indicator tubes for FEC, CMT, and MMA determination (Table 2). That the determination is correct was confirmed by the “added-found” method for specimens where ANAs were detected. In 2012, the following specimens were collected: 6 – from a gas station (route A-103); 7 and 8 – from OOO Mytischinskaya Baza (Mytischi Base Ltd.); 9 – from railroad tank No. 57366247; 10 – from OSP OOO Podolskii Produkt, Klin; 11 – from railroad tank No. 51568980. According to the laboratory liquid chromatographic determination records, the MMA volume contents in specimens 7 and 9 were 0.34 and 0.31, respectively. In determination with IT-MMA-Test, these values were 0.4 and 0.36 wt. %, respectively. To each of specimens 7 and 9 we added 0.3 wt. % of MMA, after which we determined the content of the latter using IT-MMA-Test and found it to be 0.75 and 0.72 wt. %, respectively. To specimen 6 containing 1.05 wt. % of MMA, 70 mg/dm3 of CMT, and < 7 mg/dm3 of FEC we added 0.3 wt. % of MMA and 200 mg/dm3 of FEC; the content determined after this was: MMA 1.25 wt. %, CMT 76 mg/dm3, and FEC 200 mg/dm3.
So, the proposed test methods can be used for quick monitoring of gasoline quality at the sites of their production and filling.
References
Russian Federation Patent 2425366.
Russian Federation Patent 2327157.
Russian Federation Patent 2446395.
We thank Prof. A. N. Pankratov, N. G. Chernyshevskii Saratov State University, for his advice.
Author information
Authors and Affiliations
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 5, pp. 49 – 52, September – October, 2013.
Rights and permissions
About this article
Cite this article
Ostrovskaya, V.M., Sereda, V.V., Prokopenko, O.A. et al. Indicator Tubes for Determining Anti-Knock Additives in Automotive Gasolines. Chem Technol Fuels Oils 49, 444–450 (2013). https://doi.org/10.1007/s10553-013-0468-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-013-0468-y