The feasibility of intensification of vacuum distillation of high-paraffin residual fuel oil from Kumkol crude oil (Kazakhstan) by controlling phase transitions of oil disperse systems through injection of activating additives into the residual fuel oil is investigated. Addition to residual oil of pyrolysis resin and its fractions, which differ in fractional and hydrocarbon composition, produces extreme changes in vacuum gas oil yield, and substantially contributes to shortening of distillation time. Addition of 1 wt. % of wide a pyrolysis resin fraction to residual fuel oil is highly effective.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
One means of intensifying distillation of oil and oil residues is to covert complex structural units (CCU) of an oil disperse system (ODS) from a passive to active state by introduction of activating additives in low concentrations to the feedstock, and mixing with oils of a different nature [1,2]. It is known that activating additives may increase the dissolving power of the medium, altering, moreover, the sizes of both the bubbles of the vapor phase, and also associates. According to colloidal-chemical notions, oil residues are disperse systems, the disperse phase of which is formed by low-molecular (bubbles), or high-molecular (associates) compounds [3]. Regulation of the dimensions of the CSU of the disperse phase through variation in the dissolving power of the dispersed medium by the introduction of additives may affect the properties of the system on the whole; this is reflected in variation of the yield of vacuum gasoil. Here, the minimum size of the vapor bubble characterizes one of the extremal conditions of the SCU during distillation of ODS. Moreover, when asphaltenes are dispersed in an aromatic medium, the concentration of paramagnetic centers is lowered [4].
Residual petroleum feedstock was distilled in compliance with GOST 11011– 64 in an ARN-2 plant [1,3]. An activating additive was introduced to and mixed with a preliminarily suspended feedstock. The heating rate in the initial stage of distillation (for a period of 30 min) was approximately 3 deg/min under a residual pressure of 1.33-2.66 kPa. The end point of the distillation was considered that moment when a temperature of 350-480°C was attained in the vat, after which heating was terminated.
The residual fuel oil of Kumkol high-paraffin crude (RKC) obtained from the Shumkent refinery was used as feedstock for the vacuum distillation. Pyrolysis resin (PR) from the Atyrausk Chemical Plant and its naphtha fraction with an IBP of 200°C (BPR) and median 280-380єC fraction (MFPR).
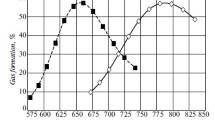
One of the most important aspects related to study of intensification of vacuum distillation of petroleum resid is exposure of the influence exerted by the concentration of various additives on regime characteristics of the process. Figure 1, a shows the dependence of the yield of vacuum gasoil on the distillation time of residual fuel oil from Kumkol crude at various BPR concentrations.
The RKC contains 16 wt. % of aromatic and 74 wt. % of paraffin-naphthene hydrocarbons, which, according to Tanashev et al. [3] govern the low dissolving power of the disperse medium [1,2]. According to Syunyaev [1,2], the low content of asphaltenes (0.82 wt. %) and relatively high resin content (10 wt. %) contribute to formation of the thickest solvate shell in the CSU system. In distilling RKC, therefore, the yield of vacuum gasoil is only 64.7 wt. %, and the distillation is continued for 40 min. Significant losses (2.9 wt. %) confirm the supposition [1,3] concerning additional energy outlays for separation of CSU into components; this is completed with partial decomposition of the CSU. According to [1,3], further boil-off of the product being distilled (7 min) suggests formation of bubbles with large critical radii.
When 1 wt. % of BRP is added to the residual fuel oil, the physico-chemical properties of the ODS are substantially changed. The BPR is a highly aromatized product, and contains a large amount of unsaturated and light aromatic hydrocarbons. The latter, increasing the dissolving power of the medium, contributes to a reduction in the surface tension on the interface between the liquid and a vapor bubble in the initial stage of its formation. According to [1,3], shortening of the initial boil-off to 4 min suggests the formation of bubbles with minimum energy outlays, wherein they are of minimum size. A reduction in the viscosity of the ODS contributes to rapid upward advancement of the bubbles. Thus, selective solution of the solvate layer of the associate is reflected in the distillation time of the activated feedstock, and in the yield of vacuum gasoil. The distillation time is therefore reduced to 19 min, and the yield of vacuum gasoil is increased to 67.5 wt. %.
Redistribution of hydrocarbons between the residue and distillate occurs at different additive concentrations, and desirable components are drawn into the vacuum distillate to some degree or other. Obviously, optimal redistribution for which the most complete entrainment of desirable fuel-oil components occurs when 1 wt. % of BPR is added to the residual fuel oil.
As is apparent from Fig. 1, a, an increase in the BPR concentration over and above 1 wt. % leads to a fractional increase in distillation time, but no greater than that for the pure residual fuel oil. A reduction in surface tension on the boundary between the liquid and forming bubbles occurs with increasing additive content in the system, resulting in reduction of pressure within the bubbles, and is reflected in the yield of the distillation products (see Table 1).
An increase in the concentration of the BRP additive over and above 1 wt. % produces a decrease in the EP of the vacuum gasoil and carbon residue of the vacuum resid, and makes it possible to suggest that some of the product being distilled remains on the surface of the supramolecular structures; this is explained by some character of the distribution of hydrocarbons between the vacuum resid and vacuum gasoil, other than by distillation without additives.
In contrast to the BPR, the MFPR consists primarily of median (34.03 wt. %) and heavy (20.8 wt. %) aromatic hydrocarbons, which are distinguished by good surface-active properties. If only native surface-active substances of the crude can be released from the associates, the MFPR would by itself participate in lowering of the surface tension of the bubbles of the vapor phase. Moreover, higher limits of the boil-off of the MFPR indicate the possibility of its participation in the distillation at higher temperatures than the BPR.
As is apparent from Fig. 1, b, the addition of 1 wt. % of MFPR and RKC accelerates the start of the boil-off of the system by up to 2 min. In that case, the residual fuel oil boils-off more rapidly than for the addition of the same amount of BPR; this is explained [1] by pronounced reduction of the system’s viscosity under the action of the MFPR, and the surface tension on the interface between the bubble and liquid, as well as by vigorous solution of the solvate shell of the CSU. A 3 wt. % increase in the yield of vacuum gasoil, and a reduction in losses to 0.9 wt % affect the formation of associates with a large nucleus (see Table 1). In our opinion , this may occur as a result of adsorption-desorption phenomena on the surface of the supramolecular structures due to variation in the specific surface energy of the CSU under the action of activating additives [1,2,4]; this also gives rise to redistribution of hydrocarbons between the distillate and distillation residue. The hydrocarbons of the dispersed medium, the dissolving power of which is highest, will interact with the surface of the supramoleular structures. Both a reduction, and also an increase in the volume of associates are possible as the composition of the dispersed medium changes due to adsorption on the surface of the CSU; this is reflected in the properties of the disperse system on the whole.
When the residual fuel oil is distilled with 1 wt. % of MFPR, the IBP of the vacuum gasoil is lower and the coking capacity of the vacuum resid higher than when the distillation takes place with 1 wt. % of BPR, suggesting the formation of a smaller vapor bubble, and associates of larger size. The required degree of dispersivity of the associates or bubbles can be regulated by external effects, and in turn, may influence the temperature of the phase transitions [1,2,4]. A further increase in the MFPR content over and above 1 wt. % in a mixture with the RKC, as in the case of the BPR, will lead to a decrease in the yield of vacuum gasoil and carbon residue of the vacuum resid; this is explained by more vigorous solution of the solvate shells, and by the formation of CSU with minimal size nuclei.
Intensification of vacuum distillation of the RKC through an increase in the dissolving power of the dispersed medium and radius of the supramolecular structures were then investigated. For this purpose, a wide pyrolysis resin fraction (PR), which is distinguished from the previous additives by the presence of resin (5.86 wt. %), and asphaltenes (2.87 wt. %) was used as an additive. The content of aromatic hydrocarbons (69.89 wt. %) in the PR contributes to an increase in the dissolving power of the dispersed medium.
As is apparent from Fig. 1c, an earlier start of boil-off of vacuum gasoil is observed when 1 wt. % of PR is added to the RKC owing to a reduction in the surface tension on the interface between the vapor–liquid phases, and in the viscosity of the system according to [1,2,5]. Where vacuum distillation of the residual fuel oil with the same amount of BPR and MFPR was continued for 19 and 21 min, respectively, after the addition of the PR, the distillation time was 28 min. This is associated with the fact that when light additives are introduced, it is primarily adjustment of the sizes of the bubbles and solution of the solvate shells of the CSU that take place, but in the presence of the PR, growth of supramolecular structures also occurs due to the asphaltenes of the additive; this is confirmed by the higher coking capacity of the vacuum resid. An increase in the content of PR over and above 1 wt. % in the residual fuel oil leads to a reduction in vacuum-gasoil yield and coking capacity of the hydrone, and also to an increase in distillation time.
Figure 2 shows the dependence of the temperature drop of the feedstock being distilled and the vapors of the fraction that is boiling off during vacuum distillation of the residual fuel oil with additives of their concentration. As is apparent, the smallest temperature drop (4є) is observed for vacuum distillation of RKC with a 1 wt. % MFPR additive. This is obviously explained by a reduction in the viscosity of the ODS to the value for which the ODS does not offer significant resistance to removal of small-size vapor bubbles from the system. The addition of 2 wt. % of MFPR produces an increase in the temperature drop to 29є. In that case, breakdown of active associates and their “disintegration” into highly disperse CSU occur. The surface of the CSU is solvated by a large part of the surface-active substances, contributing to an increase in surface tension and bubble enlargement, and a decrease in the rate of boil-off of the distillation product [1].
Uniform variation in the temperature drop during distillation of the RKC with BPR suggests that this additive is capable of changing only the size of bubbles of the vapor phase. The minimum temperature drop with the addition of 2 wt. % of BPR is associated with rapid removal of the light fraction of the additive together with the product being distilled. A further increase in temperature drop with additive content will apparently lead to a reduction in surface tension on the bubble-liquid boundary at the moment that a bubble is removed from the system; this is accompanied by vigorous growth of its size, and the resistance of the system.
As is apparent from Fig. 2, the temperature drop is greater when PR is introduced to the residual fuel oil than in the case of BPR. Playing an important role here, the resins and asphaltenes contained in the PR, which, in addition to enlarging the size of the supramolecular structures, contribute to hardening of the solvate shell when the dissolving power of the medium is high, leading to an increase in the temperature drop. It follows from the above, that activation of the residual fuel oil of the Kumkol crude by additives of a different fraction and hydrocarbon composition results in extremal variation in the yield of vacuum gasoil, and also in a pronounced reduction in the time required for vacuum distillation. In a concentration of 1 wt. % for which, in addition to an increase in yield of vacuum gasoil and shortening of the distillation time, the wide pyrolysis-resin fraction manifests the greatest activating effect, and increases the coking capacity of the vacuum resid.
References
Z. I. Syunyaev, Applied Physico-Chemical Mechanics of Oil Disperse Systems [in Russian], MINKh and GP, Moscow (1982).
Z. I. Syunyaev, R. Z. Safieva, and R. Z. Syunyaev, Oil Disperse Systems [in Russian], Khimiya, Moscow (1990).
S. T. Tanashev, U. Utbetov, Khu Ven. Tsen et al., Dokl. Natsion. Akad. Nauk Respubl. Kazakhstan, No. 1, 60–63 (2007).
Z. I. Syunyaev, Khim. Tekhnol. Topl. Masel, No. 6, 2–5 (1985).
O. F. Glagoleva and V. M. Kapustin, Technology of Oil Refining. Part 1, Primary Refinement of Crude Oil [in Russian], KolosS, Moscow (2007).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 3, pp. 33 – 36, May – June, 2013.
Rights and permissions
About this article
Cite this article
Karabaev, Z.A., Kapustin, V.M., Tanashev, S.T. et al. Intensification of Vacuum Distillation of Residual Fuel Oil from Kumkol Oil by Controlling Phase Transitions of Oil Disperse Systems. Chem Technol Fuels Oils 49, 239–244 (2013). https://doi.org/10.1007/s10553-013-0436-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-013-0436-6