Abstract
Purpose
To investigate the association between fish consumption and n-3 polyunsaturated fatty acids (n-3 PUFA) and the risk of hepatocellular carcinoma (HCC).
Methods
We identified eligible studies in MEDLINE and EMBASE up to July 2014 and the reference lists of original studies and review articles on this topic. Summary relative risks (SRR) with their 95 % confidence intervals (CI) were calculated with a random effects model.
Results
Eleven studies (three cohort studies, seven retrospective case–control studies, and one nested case–control study) met eligibility criteria. Ten articles investigated fish consumption, two articles investigated n-3 PUFA, and two articles investigated alpha-linolenic acid (ALA). The current data suggest that fish consumption was associated with 35 % reduction in HCC risk (highest vs. lowest category SRRs = 0.65, 95 % CI 0.51–0.79; test for heterogeneity p = 0.057, I 2 = 44.1 %). n-3 PUFA was associated with 51 % reduction in HCC risk (highest vs. lowest category SRRs = 0.49, 95 % CI 0.19–0.79). However, no significant inverse association was found in ALA (SRRs = 0.70, 95 % CI 0.30–1.10).
Conclusion
Our meta-analysis of observational studies provides evidence that fish consumption and n-3 PUFA has inverse association with the risk of HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a common cancer worldwide, with poor 5-year survival. An estimated 748,300 new cases and 695,900 cancer deaths occur per year, ranking it fifth among cancers for incidence and third among cancers for mortality [1]. Chronic hepatitis B (HBV) infection is the most important risk factor for HCC worldwide, especially in Asia. In Asian and African countries, more than 80 % of patients with HCC have underlying chronic HBV infection [2]. The one exception in Asia is Japan, where the prevalence of HCC has been related to chronic hepatitis C (HCV) infection [3]. In Western countries, however, chronic (HCV) infection has been determined to be present in about 60 % of patients with HCC and is the main etiologic agent leading to HCC [4, 5].
Within the context of chronic HCV and HBV infection, the presence of cirrhosis is the most important risk factor for the development of HCC. There are several modifiable risk factors in HCC, of which the most important are alcohol and tobacco [6]. Diet is also one of the most intensively studied risk factors closely related to HCC, which includes coffee and tea [7], iron [8], red and white meats [9], types of fat, selenium [10], and vitamin D [11]. However, associations with other dietary components remain unclear.
Fish plays an important role in the usual diet worldwide and is an ideal source of n-3 polyunsaturated fatty acids, which may lower cancer risk by suppressing mutations, inhibiting cellular proliferation, and inducing cell apoptosis [12, 13]. Current evidence indicated that fish consumption is inversely associated with colorectal cancer [14], lung cancer [15], and prostate cancer [16], however, not associated with breast cancer [17], gastric cancer [18], and ovarian cancer [19]. Over the past decades, so many studies have addressed the possible link between fish consumption and HCC, but the findings have been somewhat contradictory. In the present study, we therefore carried out a systematic review and meta-analysis of all available evidence of observational studies following the meta-analysis of observational studies in epidemiology guidelines [20] to clarify the association between fish consumption and risk of HCC.
Materials and methods
Data sources and searches
Two authors (M.G and K.S) independently performed a literature search using MEDLINE and EMBASE database up to 11 July 2014, and by hand searching reference lists of original studies and review articles on this topic additionally. We searched the studies with the following text words and/or medical subject heading terms: (“docosahexaenoic acid” OR “eicosapentaenoic acid” OR “docosapentaenoic acid” OR “alpha-linolenic acid” OR “polyunsaturated fatty acid” OR “omega-3 fatty acid” OR “fish”) AND (“liver cancer” OR “liver neoplasms”).
Study selection
We included studies that met all of the following criteria: (1) published as an original article; (2) using a case–control, cross-sectional, nested case–control, or cohort design; (3) the exposures of interest was n-3 polyunsaturated fatty acids and fish consumption; (4) the outcome of interest was HCC incidence or mortality; and (5) estimates of odds ratio (OR) or relative risk (RR) with corresponding 95 % confidence intervals (CIs) (or data to calculate them) for the highest versus non/lowest level of fish consumption were reported. Two authors (M.G and K.S) independently evaluated all of the studies retrieved from the databases. If there were multiple publications from the same study, the most relevant was selected, using the other publications to clarify methodology or characteristics of the population. We did not contact authors for the detailed information of primary studies.
Data extraction and quality assessment
Three authors (MJ.G, H.G, and K.L) independently evaluated all of the studies retrieved according to the prespecified selection criteria. Any discrepancies between reviewers were addressed by a joint reevaluation of the original article. The following information from each study was extracted using a standardized data collection form: the first author’s last name, year of publication, geographic location, study design, duration of follow-up, sample size, fish consumption level, the effect estimates with 95 % CIs, and covariates adjusted in the statistical analysis.
The quality of each study was assessed independently by three reviewers (MJ.G, H.G, and K.L) using the Newcastle–Ottawa Scale (NOS). The NOS consists of three parameters of quality: selection, comparability, and outcome (cohort studies) or exposure (case–control studies). The NOS assigns a maximum of four points for selection, a maximum of two points for comparability, and a maximum of three points for exposure or outcome. Any discrepancies between reviewers were addressed by a joint reevaluation of the original article.
Statistic analysis
We ignored the distinction between the various estimates of RR (i.e., OR, rate ratio, hazard ratio), and all measures were interpreted as RR for simplicity. As different studies might report different exposure categories (dichotomous, thirds, quarters, or fifths), we used the study-specific relative risk of the highest versus lowest category of fish consumption or n-3 PUFA exposure for the meta-analysis. We transformed the corresponding CIs into log RRs, and we calculated the corresponding variance with the use of the Greenland formula. For studies that lacked estimates, we calculated crude estimates from tabular data [21]. One study reported relative risk of HCC separately according to sex, age, and liver diseases condition [22]. We pooled these relative risks with a fixed effects model to get a summary relative risk of further meta-analysis. We used Woolf’s formula to evaluate the SE of the log RRs [23]. Summary relative risk (SRR) with their corresponding 95 % CIs was combined and weighted to produce pooled RRs using a random effects model.
To investigate the sources of heterogeneity across these studies, we carried out heterogeneity tests and sensitivity analysis. In heterogeneity tests, we used the Cochran Q and I 2 statistics [24], which were used to test the differences obtained between studies due to chance. For the Q statistic, a p value of less than 0.10 was considered representative of statistically significant heterogeneity. The I 2 statistic is the proportion of total variation contributed by between-study variation. It has been suggested that I 2 values of 25, 50, and 75 % are assigned to low, moderate, and high heterogeneity, respectively [25]. We conducted sensitivity analysis to estimate the influence of each individual study on the summary results by repeating the random effects meta-analysis after omitting one study at a time. We evaluated the role of several potential sources of heterogeneity by subgroup analyses according to study design, geographical locations, study quality, and adjustments for confounding variables: HBV and HCV infections, alcohol consumption, smoking, DM, and BMI.
Funnel plots and the Egger’s test were performed to test evidence of publication bias [26]. In the presence of publication bias, we used the “trim and fill” method to correct such bias [27]. Meta-analyses were carried out using STATA 12.0 (Stata Corp, College Station, TX, USA).
Result
Literature search
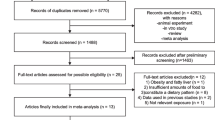
The detailed steps of our literature search are shown in Fig. 1. In brief, a total of 1,082 citations were obtained for review of title and abstract. Of the 1,082 citations, 1,048 were not relevant and five were duplicates. Hand searching the references of previous studies and systematic reviews identified four relevant studies. Full texts of the remaining 33 studies were retrieved for review. Three studies were excluded because exposure of interest was raw fish which might be contaminated by parasites [28–30]. One study was excluded because raw data were not applicable to calculate them [31]. Finally, 11 studies were included in the meta-analysis. (Fig. 1).
Study characteristics
Eleven articles that met our inclusion criteria in this meta-analysis were published between 1988 and 2013. There were three cohort studies [22, 32, 33] with mean follow-up time ranging from 9 to 11.4 years, seven retrospective case–control studies [12, 21, 34–38], and one nested case–control study [9]. Ten articles described the association between fish consumption and risk of HCC [9, 12, 21, 22, 32–35, 37, 38], two described the association between n-3 PUFA or ALA intake and risk [32, 36], and one reported the association between EPA, DHA, DPA intake and risk [32]. The average score for the quality assessment of included studies was 8.1 (high quality) (Table 1).
Meta-analysis
Meta-analysis of ten studies in a random effects model found that fish consumption decreased the risk of HCC by 35 %, with moderate heterogeneity among studies (SRRs = 0.65, 95 % CI 0.51–0.79; test for heterogeneity p = 0.057, I 2 = 44.1 %) (Fig. 2).
In a sensitivity analysis, the overall homogeneity and effect size were calculated by removing one study at a time. We found that there were no changes in the direction of effect when any one study was excluded, supporting the stability of the inverse association between fish consumption and risk of HCC.
We subsequently conducted subgroup systematic review and meta-analysis according to study design, geographical locations, study quality, and adjustments for confounding variables: HBV and HCV infections, alcohol consumption, smoking, DM, and BMI (Table 2). In stratified analysis by geographical locations, a significant inverse association between fish consumption and risk of HCC was found for studies conducted in both Europe (SRRs = 0.68; 95 % CI 0.41–0.94; p heterogeneity = 0.025, I 2 = 64.2 %) and Asia (SRRs = 0.58; 95 % CI 0.42–0.75; p heterogeneity = 0.514, I 2 = 0 %). However, no significant inverse association was found in America (SRRs = 0.86; 95 % CI 0.62–1.10).
The SRRs were statistically significant in nested case–control studies (SRRs = 0.59; 95 % CI 0.29–0.88), cohort studies (SRRs = 0.73; 95 % CI 0.56–0.90; p heterogeneity = 0.451, I 2 = 0 %), and population-based case–control studies (SRRs = 0.54; 95 % CI 0.15–0.93; p heterogeneity = 0.098, I 2 = 63.6 %). However, no significant inverse association was found in hospital-based case–control studies (SRRs = 0.71; 95 % CI 0.35–1.06; p heterogeneity = 0.012, I 2 = 72.4 %) (Table 2).
In stratified analysis by study quality, significant inverse associations were found for NOS > 7 (SRRs = 0.71; 95 % CI 0.54–0.88; p heterogeneity = 0.107, I 2 = 42.6 %) and NOS 2 7 (SRRs = 0.55; 95 % CI 0.33–0.77; p heterogeneity = 0.221, I 2 = 31.9 %).
Hepatitis B virus and HCV infections, alcohol consumption, smoking, DM, and BMI are important confounders for risk of HCC. When we limited the meta-analysis to studies that controlled for one of the above confounders, significant inverse associations could still be found in adjustment for HBV and HCV infections (n = 3, SRRs = 0.47; 95 % CI 0.20–0.74; p heterogeneity = 0.390), alcohol consumption (n = 6, SRRs = 0.64; 95 % CI 0.49–0.80; p heterogeneity = 0.039), smoking (n = 6, SRRs = 0.63; 95 % CI 0.47–0.80; p heterogeneity = 0.026), and BMI (n = 4, SRRs = 0.78; 95 % CI 0.58–0.98; p heterogeneity = 0.179). However, no significant inverse association was found in adjustment for DM (n = 3, SRRs = 0.54; 95 % CI 0.04–1.05) (Table 2).
Two studies reported the association between n-3 PUFA and risk of HCC, n-3 PUFA was inversely associated with risk (SRRs = 0.49, 95 % CI 0.19–0.79; test for heterogeneity p = 0.929, I 2 = 0 %) (Fig. 3). Two studies reported the association between ALA and risk of HCC, ALA was not associated with risk (SRRs = 0.70, 95 % CI 0.30–1.10; test for heterogeneity p = 0.999, I 2 = 0 %) (Fig. 3). Only one study reported EPA, DPA, DHA, and risk of HCC, and hazard ratios for the highest vs lowest category were 0.55 (95 % CI 0.22–1.39) for EPA, 0.55 (95 % CI 0.21–1.42) for DPA, and 0.59 (95 % CI 0.22–1.57) for DHA in subjects who were anti-HCV or HBsAg positive.
Publication bias
Slight publication bias was observed in the literature based on the Begg’s test (p = 0.04) (Fig. 4) and Egger’s regression test (p < 0.01). Trim and fill analysis, however, did not change the result (SRRs = 0.69, 95 % CI 0.52–0.87), suggesting that the effect of publication bias could be negligible.
Discussion
In this meta-analysis, fish consumption may decrease the risk of HCC by as much as 35 %. Dietary intake of n-3 PUFA, but not alpha-linolenic acid (ALA), was associated with a lower risk of HCC. This is the first meta-analysis summarized the evidence to date regarding the association between fish consumption and n-3 PUFA and risk of HCC.
The inverse association between fish consumption and risk of HCC is biologically plausible. Consumption of fish provides high-quality protein, unsaturated essential fatty acids, as well as certain vitamins and minerals. Using animal models, researchers have found that supplementing the diet of tumor-bearing mice or rats with purified n-3 fatty acids has slowed the growth of HCC [39]. Larsson summarized current knowledge of the potential mechanisms of the anti-carcinogenic actions of n-3 fatty acids : (1) suppression of arachidonic acid-derived eicosanoid biosynthesis, (2) influence on transcription factor activity, gene expression, and signal transduction, (3) alteration of estrogen metabolism, (4) increased or decreased production of free radicals and reactive oxygen species, and (5) effect on insulin sensitivity and membrane fluidity [40].
There may be reasons for the discrepancies observed between included studies. First, self-reported dietary intake (especially via food frequency questionnaire) is notoriously poor and plagued by problems of random error and systematic error associated with participant characteristics. Second, the protective effect of fish consumption on HCC risk may be counterbalanced by the negative effect of contaminants. Among contaminants found in fish are methylmercury [41], polychlorinated dibenzo-p-dioxins [42], dibenzofurans, organochlorine residues, and other chemicals. These chemicals have high toxicity and carcinogenic potency, and a few epidemiological studies suggested that pesticides and some of these chemicals may be related to HCC [43]. Third, variation in cooking methods across study populations on these studies may have contributed to the inconsistent findings. Heterocyclic amines and polycyclic aromatic hydrocarbons formed during preparation of the fish at high temperatures may be one of the reasons. Fourth, fish is also a source of n-6 fatty acids, which can enhance growth and promotion of breast cancer, pancreatic cancer, and prostate cancer [44–46]. But there is still few experimental or clinical evidence about n-6 fatty acids intake and the risk of HCC. Fresh water fish contain lower levels of n-3 fatty acids but higher levels of n-6 fatty acids than marine fish. Most of the studies included in our meta-analysis, however, did not specify what type of fish was consumed. Fifth, only 3 of 10 studies in this meta-analysis controlled for HBV and HCV infections. The preexisting liver disease, such as hepatitis and cirrhosis, occurs well before HCC, which may affect dietary intake of fatty acid. The protective effects of n-3 PUFA may be underestimated.
There was significant heterogeneity observed across studies, but the heterogeneity is moderate and acceptable with I 2 = 44.1 %, so we could still be able to combine studies in a meta-analysis. We analyzed this review in both fixed effects and random effects and they varied a little. The more conservative one, random effects, was chosen finally. When we tried to carry out subgroup analysis according to study design, geographical locations, study quality, and adjustments to investigate sources of heterogeneity, statistical heterogeneity was lower in Asian group and cohort studies. This suggests that study design and geographical locations might account for heterogeneity observed.
Our meta-analysis has several strengths. (1) Studies were included after a comprehensive and systematic search of the literature by using an extensive search strategy. (2) The majority of the included studies evaluated multiple confounders including HBV and HCV infections, alcohol consumption, smoking, DM, and BMI. (3) With available evidence and enlarged number of studies to date, we have enhanced statistical power to detect any associations between fish consumption and risk of HCC.
Our meta-analysis has limitations that affect interpretation of the true results. First, seven of 11 studies in this meta-analysis used case–control design, which was more susceptible to recall and selection biases than a cohort design. On the other hand, cohort studies may be affected by detection bias. Second, there is substantial heterogeneity across studies. The heterogeneity was likely due to the variation in exposure definitions, exposure ranges, and population characteristics between studies. Third, unmeasured or uncontrolled confounding inherited from original studies is a concern in this meta-analysis. Most estimate risks were derived from multivariable models, but individual studies did not adjust for potential confounding factors in a consistent way.
In summary, our meta-analysis of observational studies provides evidence that fish consumption and n-3 PUFA has inverse association with the risk of HCC. Given the small number of studies included in this meta-analysis, further prospective cohort studies with larger sample size, well controlled for confounding factors, and more accurate assessment of fish consumption and n-3 PUFA are needed to affirm the effect of fish on HCC.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- BMI:
-
Body mass index
- DM:
-
Diabetes mellitus
- PUFA:
-
Polyunsaturated fatty acids
- ALA:
-
Alpha-linolenic acid
- EPA:
-
Eicosapentaenoic acid
- DPA:
-
Docosapentaenoic acid
- DHA:
-
Docosahexaenoic acid
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. doi:10.3322/caac.20107
Beasley RP (1988) Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer 61(10):1942–1956
Shiratori Y, Shiina S, Imamura M, Kato N, Kanai F, Okudaira T, Teratani T, Tohgo G, Toda N, Ohashi M, Et A (1995) Characteristic difference of hepatocellular carcinoma between hepatitis B- and C-viral infection in Japan. Hepatology 22(4 Pt 1):1027–1033
Altekruse SF, McGlynn KA, Reichman ME (2009) Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 27(9):1485–1491. doi:10.1200/JCO.2008.20.7753
El-Serag HB, Rudolph KL (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132(7):2557–2576. doi:10.1053/j.gastro.2007.04.061
Purohit V, Rapaka R, Kwon OS, Song BJ (2013) Roles of alcohol and tobacco exposure in the development of hepatocellular carcinoma. Life Sci 92(1):3–9. doi:10.1016/j.lfs.2012.10.009
Montella M, Polesel J, La Vecchia C, Dal Maso L, Crispo A, Crovatto M, Casarin P, Izzo F, Tommasi LG, Talamini R, Franceschi S (2007) Coffee and tea consumption and risk of hepatocellular carcinoma in Italy. Int J Cancer 120(7):1555–1559. doi:10.1002/ijc.22509
Kew MC (2014) Hepatic iron overload and hepatocellular carcinoma. Liver Cancer 3(1):31–40. doi:10.1159/000343856
Fedirko V, Trichopolou A, Bamia C, Duarte-Salles T, Trepo E, Aleksandrova K, Nothlings U, Lukanova A, Lagiou P, Boffetta P, Trichopoulos D, Katzke VA, Overvad K, Tjonneland A, Hansen L, Boutron-Ruault MC, Fagherazzi G, Bastide N, Panico S, Grioni S, Vineis P, Palli D, Tumino R, Bueno-de-Mesquita HB, Peeters PH, Skeie G, Engeset D, Parr CL, Jakszyn P, Sanchez MJ, Barricarte A, Amiano P, Chirlaque M, Quiros JR, Sund M, Werner M, Sonestedt E, Ericson U, Key TJ, Khaw KT, Ferrari P, Romieu I, Riboli E, Jenab M (2013) Consumption of fish and meats and risk of hepatocellular carcinoma: the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann Oncol 24(8):2166–2173. doi:10.1093/annonc/mdt168
Bettinger D, Schultheiss M, Hennecke N, Panther E, Knuppel E, Blum HE, Thimme R, Spangenberg HC (2013) Selenium levels in patients with hepatitis C virus-related chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma: a pilot study. Hepatology 57(6):2543–2544. doi:10.1002/hep.26142
Fedirko V, Duarte-Salles T, Bamia C, Trichopoulou A, Aleksandrova K, Trichopoulos D, Trepo E, Tjonneland A, Olsen A, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, Kvaskoff M, Kuhn T, Lukanova A, Boeing H, Buijsse B, Klinaki E, Tsimakidi C, Naccarati A, Tagliabue G, Panico S, Tumino R, Palli D, Bueno-de-Mesquita HB, Siersema PD, Peters PH, Lund E, Brustad M, Standahl OK, Weiderpass VE, Zamora R, Sanchez MJ, Ardanaz E, Amiano P, Navarro C, Quiros JR, Werner M, Sund M, Lindkvist B, Malm J, Travis RC, Khaw KT, Stepien M, Scalbert A, Romieu I, Lagiou P, Riboli E, Jenab M (2014) Pre-diagnostic circulating vitamin D levels and risk of hepatocellular carcinoma in European populations: a nested case–control study. Hepatology. doi:10.1002/hep.27079
Fernandez E, Chatenoud L, La Vecchia C, Negri E, Franceschi S (1999) Fish consumption and cancer risk. Am J Clin Nutr 70(1):85–90
Rose DP, Connolly JM (1999) Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther 83(3):217–244
Wu S, Feng B, Li K, Zhu X, Liang S, Liu X, Han S, Wang B, Wu K, Miao D, Liang J, Fan D (2012) Fish consumption and colorectal cancer risk in humans: a systematic review and meta-analysis. Am J Med 125(6):551–559. doi:10.1016/j.amjmed.2012.01.022
Song J, Su H, Wang BL, Zhou YY, Guo LL (2014) Fish consumption and lung cancer risk: systematic review and meta-analysis. Nutr Cancer 66(4):539–549. doi:10.1080/01635581.2014.894102
Szymanski KM, Wheeler DC, Mucci LA (2010) Fish consumption and prostate cancer risk: a review and meta-analysis. Am J Clin Nutr 92(5):1223–1233. doi:10.3945/ajcn.2010.29530
Zheng JS, Hu XJ, Zhao YM, Yang J, Li D (2013) Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ 346:f3706
Wu S, Liang J, Zhang L, Zhu X, Liu X, Miao D (2011) Fish consumption and the risk of gastric cancer: systematic review and meta-analysis. BMC Cancer 11:26. doi:10.1186/1471-2407-11-26
Jiang PY, Jiang ZB, Shen KX, Yue Y (2014) Fish intake and ovarian cancer risk: a meta-analysis of 15 case–control and cohort studies. PLoS One 9(4):e94601. doi:10.1371/journal.pone.0094601
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283(15):2008–2012
La Vecchia C, Negri E, Decarli A, D’Avanzo B, Franceschi S (1988) Risk factors for hepatocellular carcinoma in northern Italy. Int J Cancer 42(6):872–876
Kurozawa Y, Ogimoto I, Shibata A, Nose T, Yoshimura T, Suzuki H, Sakata R, Fujita Y, Ichikawa S, Iwai N, Fukuda K, Tamakoshi A (2004) Dietary habits and risk of death due to hepatocellular carcinoma in a large scale cohort study in Japan. Univariate analysis of JACC study data. Kurume Med J 51(2):141–149
Greenland S (1987) Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9:1–30
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. doi:10.1002/sim.1186
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. doi:10.1136/bmj.327.7414.557
Sterne JA, Egger M (2001) Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 54(10):1046–1055
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56(2):455–463
Lu SN, Lin TM, Chen CJ, Chen JS, Liaw YF, Chang WY, Hsu ST (1988) A case–control study of primary hepatocellular carcinoma in Taiwan. Cancer 62(9):2051–2055
Qiu XQ, Tan SK, Yu HP, Zeng XY, Li LQ, Hu L (2008) Synergistic effect of HBV infection, alcohol and raw fish consumption on oncogenisis of primary hepatic carcinoma. Zhonghua Zhong Liu Za Zhi 30(2):113–115
Tan SK, Qiu XQ, Yu HP, Zeng XY, Xiao ZM, Hu L (2008) Etiologic fraction and interaction of risk factors for primary hepatic carcinoma in Guangxi, China. Zhonghua Yu Fang Yi Xue Za Zhi 42(3):169–172
Kuper H, Tzonou A, Lagiou P, Mucci LA, Trichopoulos D, Stuver SO, Trichopoulou A (2000) Diet and hepatocellular carcinoma: a case–control study in Greece. Nutr Cancer 38(1):6–12. doi:10.1207/S15327914NC381_2
Sawada N, Inoue M, Iwasaki M, Sasazuki S, Shimazu T, Yamaji T, Takachi R, Tanaka Y, Mizokami M, Tsugane S (2012) Consumption of n-3 fatty acids and fish reduces risk of hepatocellular carcinoma. Gastroenterology 142(7):1468–1475. doi:10.1053/j.gastro.2012.02.018
Daniel CR, Cross AJ, Graubard BI, Hollenbeck AR, Park Y, Sinha R (2011) Prospective investigation of poultry and fish intake in relation to cancer risk. Cancer Prev Res (Phila) 4(11):1903–1911. doi:10.1158/1940-6207.CAPR-11-0241
Wang MP, Thomas GN, Ho SY, Lai HK, Mak KH, Lam TH (2011) Fish consumption and mortality in Hong Kong Chinese–the LIMOR study. Ann Epidemiol 21(3):164–169. doi:10.1016/j.annepidem.2010.10.010
Kanazir M, Boricic I, Delic D, Tepavcevic DK, Knezevic A, Jovanovic T, Pekmezovic T (2010) Risk factors for hepatocellular carcinoma: a case–control study in Belgrade (Serbia). Tumori 96(6):911–917
Polesel J, Talamini R, Montella M, Maso LD, Crovatto M, Parpinel M, Izzo F, Tommasi LG, Serraino D, La Vecchia C, Franceschi S (2007) Nutrients intake and the risk of hepatocellular carcinoma in Italy. Eur J Cancer 43(16):2381–2387. doi:10.1016/j.ejca.2007.07.012
Talamini R, Polesel J, Montella M, Dal Maso L, Crispo A, Tommasi LG, Izzo F, Crovatto M, La Vecchia C, Franceschi S (2006) Food groups and risk of hepatocellular carcinoma: a multicenter case–control study in Italy. Int J Cancer 119(12):2916–2921. doi:10.1002/ijc.22267
Yu SZ, Huang XE, Koide T, Cheng G, Chen GC, Harada K, Ueno Y, Sueoka E, Oda H, Tashiro F, Mizokami M, Ohno T, Xiang J, Tokudome S (2002) Hepatitis B and C viruses infection, lifestyle and genetic polymorphisms as risk factors for hepatocellular carcinoma in Haimen, China. Jpn J Cancer Res 93(12):1287–1292
Metwally NS, Kholeif TE, Ghanem KZ, Farrag AR, Ammar NM, Abdel-Hamid AH (2011) The protective effects of fish oil and artichoke on hepatocellular carcinoma in rats. Eur Rev Med Pharmacol Sci 15(12):1429–1444
Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A (2004) Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr 79(6):935–945
Sanzo JM, Dorronsoro M, Amiano P, Amurrio A, Aguinagalde FX, Azpiri MA (2001) Estimation and validation of mercury intake associated with fish consumption in an EPIC cohort of Spain. Public Health Nutr 4(5):981–988
Sjodin A, Hagmar L, Klasson-Wehler E, Bjork J, Bergman A (2000) Influence of the consumption of fatty baltic sea fish on plasma levels of halogenated environmental contaminants in Latvian and Swedish men. Environ Health Perspect 108(11):1035–1041
Rojas E, Herrera LA, Poirier LA, Ostrosky-Wegman P (1999) Are metals dietary carcinogens? Mutat Res 443(1–2):157–181
Williams CD, Whitley BM, Hoyo C, Grant DJ, Iraggi JD, Newman KA, Gerber L, Taylor LA, McKeever MG, Freedland SJ (2011) A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr Res 31(1):1–8. doi:10.1016/j.nutres.2011.01.002
Funahashi H, Satake M, Hasan S, Sawai H, Newman RA, Reber HA, Hines OJ, Eibl G (2008) Opposing effects of n-6 and n-3 polyunsaturated fatty acids on pancreatic cancer growth. Pancreas 36(4):353–362. doi:10.1097/MPA.0b013e31815ccc44
Tokudome S, Kuriki K, Suzuki S, Nahomi I, Goto C, Tokudome Y, Takeda E, Ichikawa Y, Okuyama H (2003) n-6 Polyunsaturated fatty acids and breast cancer. Nutr Cancer 47(2):210–212. doi:10.1207/s15327914nc4702_14
Acknowledgments
The authors’ responsibilities were as follows—LS and LN: contributed to the study design and writing of the manuscript; GM and SK: contributed to the data search and data collection; GMJ, GH, and LK: contributed to the data extraction and quality assessment; and YCC: contributed to the data analysis.
Conflict of interest
This work was supported by a grant from the International S&T Cooperation Program of China (No. 2013DFA11150) and the Plan Project of Science & Technology from Jining city (2014JNNK21&2014JNYYF03). The authors have declared that no competing interests exist.
Author information
Authors and Affiliations
Corresponding author
Additional information
Min Gao and Kai Sun have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Gao, M., Sun, K., Guo, M. et al. Fish consumption and n-3 polyunsaturated fatty acids, and risk of hepatocellular carcinoma: systematic review and meta-analysis. Cancer Causes Control 26, 367–376 (2015). https://doi.org/10.1007/s10552-014-0512-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-014-0512-1