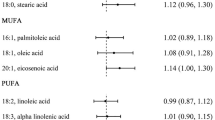
Abstract
Background
Growing body of laboratory evidence supports the beneficial effects of n-3 polyunsaturated fatty acids (PUFAs) on colorectal cancer (CRC) prevention. Epidemiologic studies investigating the relationship between n-3 PUFAs intake and risk of CRC, however, have been inconsistent. We aimed to clarify the relation by conducting a meta-analysis of prospective studies.
Methods
Eligible studies were identified by searching PubMed database and by carefully reviewing bibliographies of retrieved publications. Summary relative risks (RRs) with their 95 % confidence intervals (CIs) were computed with a random-effects model. Subgroup, meta-regression, and dose–response analyses were performed to explore potential sources of heterogeneity.
Results
A total of 14 prospective studies involving 8,775 cancer cases were included in the final analysis. Overall, total n-3 or marine PUFAs intake was not associated with risk of CRC (RR 0.99 and 1.00). However, there was a trend toward reduced risk of proximal colon cancer (total n-3 PUFAs: RR 0.83, 95 % CI 0.66–1.05; marine PUFAs: RR 0.81, 95 % CI 0.59–1.10) and a significant increased risk of distal colon cancer (total n-3 PUFAs: RR 1.26, 95 % CI 1.06–1.50; marine PUFAs: RR 1.38, 95 % CI 1.11–1.71). Furthermore, marine PUFAs intake accessed longer before diagnosis was associated 21 % reduced risk of CRC (RR 0.79, 95 % CI 0.63–1.00).
Conclusion
Overall, this meta-analysis finds no relation between n-3 PUFAs intake and risk of CRC. The observed subsite heterogeneity within colon cancer and the possible effect modification by latency time merit further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third common cancer in males and the second in females [1]. Diet and lifestyle factors play an important role in the etiology of CRC, and a large proportion of CRC incidence might be prevented by a healthy lifestyle [2, 3]. However, a limited number of dietary factors have been identified to prevent CRC.
N-3 polyunsaturated fatty acids (PUFAs) are of particular interest given that emerging laboratory evidence shows their anti-inflammatory and anticarcinogenic effects. In addition, cumulative epidemiologic evidence supports beneficial effects of fish, a major dietary source of n-3 PUFAs, on CRC prevention [4, 5]. Animal [6] and prospective epidemiologic studies [7], together with clinical trials [8, 9], suggest that n-3 PUFAs decrease release of inflammatory biomarkers in rat and in human. Cancer-related inflammation is widely accepted to promote tumor growth and progression through induction of gene mutations, inhibition of apoptosis, and stimulation of angiogenesis and cell proliferation [10, 11]. Thus, intake of n-3 PUFAs is biologically plausible to reduce CRC incidence. Other proposed mechanisms whereby n-3 PUFAs may prevent CRC have been well documented in the review by Larsson et al. [12], including impacts on transcription factor activity, gene expression, signal transduction, production of free radicals and reactive oxygen species, and insulin sensitivity, etc.
Despite the above-mentioned evidence, prospective studies investigating the association of n-3 PUFAs intake with risk of CRC have yielded inconsistent findings. Two meta-analyses [5] have been conducted to clarify the relation, with the more recent one [13] which included only seven cohorts suggesting possible sex differences, with a borderline significant inverse association in men (RR 0.87). However, seven other prospective studies were not available or missed at that time. In an attempt to further elucidate the relationship between n-3 PUFAs intake and risk of CRC, a meta-analysis of prospective studies was carried out.
Materials and methods
Literature search
We performed a literature search through 10 October 2014 on PubMed database using the search strategy reported in Supplementary figure 1, which involved cancer of colorectum and its subsite, n-3 PUFAs and corresponding subtypes including α-linolenic acid (ALA), docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and docosapentaenoic acid (DPA), as well as design of studies. The bibliographies of retrieved publications and previous meta-analyses were also carefully reviewed to identify further studies.
Study selection
We considered the studies fulfilling the following criteria: (1) prospective study design; (2) n-3 PUFAs (or subtypes) as the exposure of interest; (3) colorectal (or colon or rectal) cancer as the outcome of interest; and (4) consideration of odds ratio (OR) or relative risk (RR) or hazard ratio (HR) estimates with corresponding 95 % confidence interval (CI). In cases of multiple published publications from the same study, the one with the largest events was included.
Data extraction
Using a standardized data-collection form, the following data were extracted from each included study: the first author’s last name, publication year, country of origin, average length of follow-up, sex of participants, number of cases and participants, levels of exposure and adjusted OR, RR, or HR of CRC (with corresponding 95 % CI for each category of exposure), and variables accounted for in the statistical model. Literature search, study selection, and data extraction were conducted independently by two authors, with any disagreement resolved by consensus.
Statistical analysis
A DerSimonian and Laird [14] random-effects model, which considers both within-study and between-study variations, was assigned to calculate the summary risk estimates. The reported ORs and HRs in the primary studies were considered as RR approximations because the incidence of CRC was sufficiently low for the rare disease assumption to apply. Any results separately reported by cancer stage [15], cancer sites [16], or by different types of n-3 PUFAs [17] were combined with the fixed-effects model, and the combined results were used in the primary analysis. Given the possible sex differences [13], males and females were treated as two independent cohort samples in the studies that only reported sex-specific results. If both the results for dietary and total (dietary plus supplements) intake were reported, as in one study [18], the results for total intake were included in the meta-analysis.
Our primary analyses considered two different types of PUFAs, namely total n-3 PUFAs (including ALA, DHA, EPA, and DPA) and marine PUFAs (including DHA, EPA, and DPA). We considered the intake as total n-3 PUFAs intake if the authors only presented intake levels but did not provide information about which types of n-3 PUFAs were included. For one study [19] stating that intake of n-3 PUFAs was calculated according to frequency of fish/shellfish intake, we considered the intake as marine PUFAs intake.
To explore potential sources of heterogeneity, we conducted stratified and meta-regression analyses according to predefined factors including: sex, geographic region, average duration of follow-up, cancer sites within colorectum/colon, and specific types of n-3 PUFAs. In addition, we further performed sex-specific subgroup analysis to investigate possible sex differences.
Given that n-3 PUFAs intake in the highest and lowest categories differed substantially between studies, we also conducted a dose–response analysis by use of the method proposed by Greenland and Longnecker [20] and Orsini et al. [21]. This method requires that the number of cases and person-years and the risk estimates with their variance estimates for at least three quantitative exposure categories are known. For the studies that did not provide the number of cases and person-years in each exposure category, we estimated these data from total number of cases and person-years. For each study, the median or mean level of intake for each category was assigned to each corresponding RR estimate. When the median or mean intake per category was not provided, we assigned the midpoint of the upper and lower boundaries in each category as average intake. If the highest or lowest category was open-ended, we assumed the width of the interval to be the same as in the closest category. If the intakes were presented in densities (i.e., g/1,000 kcal), as in two studies [15, 22], we recalculated the intakes to absolute intakes using the mean or median energy intake.
To characterize the shape of the association, we also examined a potential linear/nonlinear relationship between n-3 fatty acids and CRC by modeling exposure levels using restricted cubic splines with three knots at percentiles 10, 50, and 90 % of the distribution [23]. The p value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline is equal to zero.
Heterogeneity test was performed using Q and I 2 statistics [24]. For the Q statistic, p < 0.1 was considered as statistically significant, and for the I 2, a value of >50 % was considered severe heterogeneity. Potential publication bias was investigated using Egger’s regression asymmetry test [25]. All statistical analyses were performed using STATA software, version 11.0 (STATA Corp., College Station, TX, USA). All p values were two-sided, and the level of significance was at <0.05, unless explicitly stated.
Results
Study characteristics
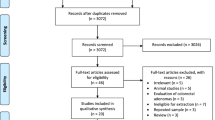
A flow chart of study screening and selection process is reported in Fig. 1. In sum, a total of 585 citations were identified through the primary search, and two additional articles were found by reviewing reference lists. After screening the titles and abstracts, 22 potentially relevant articles were selected for full-text review, of which seven were excluded because they did not investigate n-3 PUFAs or CRC, or did not report RR estimates. We further excluded two publications [26, 27] because they were duplicate reports of other two [15, 16] with larger events. Finally, 14 eligible prospective studies [15–19, 22, 28–34] (results of two independent cohorts were presented in one publication [34]) were included in the meta-analysis.
The characteristics of each study are summarized in Supplementary Table 1. The 14 studies were published between 1994 and 2014 and covered a total of 8,775 cancer cases and 731,555 participants. These studies were from the USA (n = 7), Japan (n = 2), the UK (n = 1), Finland (n = 1), Sweden (n = 1), China (n = 1), and Singapore (n = 1). All studies had reported multivariable-adjusted risk estimates. The levels of n-3 PUFAs intake varied substantially across studies.
Total n-3 fatty acids
Ten studies [15–17, 28–31, 33, 34] reported results for the association of total n-3 PUFAs intake and risk of CRC. The summary RR for the highest compared with lowest intake was 0.99 (95 % CI 0.92–1.06) (Fig. 2), suggesting that intake of total PUFAs was not associated with risk of CRC. We observed little evidence of heterogeneity (p = 0.34, I 2 = 10.5 %) or publication bias (p for Egger’s test = 0.61).
Marine fatty acids
A total of 11 studies [15–19, 22, 29, 31, 32, 34] were included in the meta-analysis of marine PUFAs intake and risk of CRC. A pooled analysis of these studies yielded a summary RR of 1.00 (95 % CI 0.93–1.07) (Fig. 3). There was no evidence of heterogeneity (p = 0.51, I 2 = 0.0 %) or publication bias (p for Egger’s test = 0.73).
Subgroup and sensitivity analysis
Table 1 presents the results of the stratified analyses performed to explore potential sources of heterogeneity. The null association of total n-3 or marine PUFAs with risk of CRC was consistently observed in most strata. However, some evidence of subsite heterogeneity within colon cancer was found (p for interaction <0.05). There was a trend toward reduced risk of proximal colon cancer for subjects with higher intake of n-3 (RR 0.83, 95 % CI 0.66–1.05) and marine PUFAs (RR 0.81, 95 % CI 0.59–1.10); conversely, higher intake of total n-3 and marine PUFAs was, respectively, associated with a 26 % (RR 1.26, 95 % CI 1.06–1.50) and 38 % (RR 1.38, 95 % CI 1.11–1.71) significant increase in the risk of distal colon cancer.
Considering possible sex differences, we further conducted subgroup analysis defined by sex (Table 2). Except for a significant positive association between marine PUFAs intake and risk of CRC among Asian men (RR 1.23, 95 % CI 1.02–1.49), there was no relation observed in other strata.
There were four studies that also investigated the association of marine PUFAs intake with risk of CRC by latency time between intake and the clinical diagnosis of CRC. By analyzing these studies, we found that marine PUFAs intake accessed 10–13 years or longer before diagnosis was associated 21 % reduction in the risk of CRC (RR 0.79, 95 % CI 0.63–1.00, Fig. 4).
Meta-analysis of marine n-3 polyunsaturated fatty acids intake (high vs. low intake) and risk of colorectal cancer by latency time between intake and diagnosis of cancer. The NHS study and the HPFS study included three groups for the “<12 years” lag, and we pooled these results with the fixed-effect model and included the pooled results in this analysis. M male, F female, NHS Nurses’ Health Study, HPFS Health Professionals Follow-Up Study
Dose–response analysis
One study [19] investigating marine PUFAs was not eligible for this analysis because the levels of intake were not reported. We found no evidence of a linear dose–response association between intake of n-3 or marine PUFAs and CRC. The summary RR for an increase in n-3 PUFAs intake of 1,000 mg/day was 0.96 (95 % CI 0.89–1.04), and the summary RR for an increase in marine PUFAs intake of 500 mg/day was 1.03 (95 % CI 0.97–1.11). There was no heterogeneity among studies (p > 0.50, I 2 = 0.0 %).
There was a suggestion of a nonlinear association between total n-3 PUFAs intake and CRC risk (p for nonlinearity = 0.063, Fig. 5). Further analysis by sex (Supplementary figure 2) explored that this observation was driven by female data (p for nonlinearity = 0.037). In women, low-to-moderate intake of total n-3 PUFAs was associated with a weak but significant increased risk of CRC. Very high intake tended to reduce the risk, but data points became especially few at these levels. For marine PUFAs, there was no evidence of nonlinearity, even when stratifying by sex (p for nonlinearity >0.20, figure not shown).
Discussion
In this meta-analysis of prospective studies, we found no overall association between intake of n-3 PUFAs and risk of CRC. The results were similar for total n-3 and marine PUFAs, and did not vary according to several predefined study and population characteristics.
Heterogeneity emerged in the analysis of anatomical subsite of colon cancer, both for total n-3 and for marine PUFAs, with high intake nonsignificantly reducing risk of proximal colon cancer and significantly increasing risk of distal colon cancer. There was also evidence of possible effect modification by time, with marine PUFAs intake 10–13 years or longer before clinical detection of CRC reducing the risk. We were not able to confirm the observation of reduced risk in men reported in the recent meta-analysis [13], and there was even an increased risk in Asian men in our analysis.
Our findings contradicted evidence from laboratory studies. Of note, the doses of n-3 PUFAs used in the laboratory studies are much higher than human intake. Thus, it is possible that there is a threshold for n-3 fatty acids to protect CRC. This possibility was somewhat supported by the results of our dose–response analysis, in which a trend toward reduced risk was observed when intake was very high (Fig. 5). We also observed a slightly increased risk in women when the intake of n-3 PUFAs was relatively low (Supplementary figure 2). We cannot explain this observation because a potentially beneficial nutrient is biologically unlikely to increase cancer risk in low levels and decrease risk in high levels, and this result may have occurred by chance given the multiple sub-analyses performed. Of note is that long-term exposure of n-3 PUFAs should be considered in the context of the whole dietary pattern. Epidemiologic evidence supports the benefits of fish, a main dietary source of n-3 PUFAs, on CRC protection. However, even if n-3 PUFAs are one of the causal components in fish, it is also plausible that its intake as part of a matrix of other nutrients in fish or other foods may be essential for its benefits.
On the other hand, there was also evidence pointing to adverse effects of n-3 PUFAs or fish intake on CRC development. Dietary n-3 PUFAs have been reported to promote colon cancer metastasis in rat liver [35]. Intake of specific fish (e.g., smoked and salted fish) has been suggested to increase CRC risk [36], possibly due to N-nitroso compounds (NOCs) produced during preparations [36, 37]. This might in part explain the increased risk for men in Asian (Table 2), where smoked and salted fish are commonly consumed.
Our finding of the subsite heterogeneity within colon cancer appears biologically plausible. It has been shown that the production of short-chain PUFAs resulting from fermentation reactions are higher in proximal than distal colon [38]. In addition, the pro-mutagenic lesion O(6)-methyldeoxyguanosine, a marker of exposure to NOCs, was found to be lower in normal proximal than in distal colonic DNA in patients with CRC [39]. Therefore, n-3 PUFAs may insert protection against CRC in the proximal colon mucosa, while high NOCs may dilute or even reverse the protection on distal colon cancer. Given the small number of studies distinguishing between proximal and distal colon cancer, future prospective studies are warranted to confirm our results, and more laboratory data are also needed to further explore potential mechanisms involved.
Our finding of possible modification by time suggests early-acting beneficial effects of marine PUFAs on CRC development. Marine PUFAs have been experimentally suggested to inhibit initiation of colon cancer in mice [40] and epidemiologically found to be associated with reduced risk of colorectal adenoma [41–43]. All together, these observations indicate that the benefits of n-3 PUFAs might be restricted to the early stage of CRC.
This meta-analysis presents several strengths. The prospective design of original studies eliminated the possibility of recall and selection biases. The large number of cases involved enhanced the statistical power. Conducting various analyses enabled us to comprehensively understand the association of n-3 PUFAs (and their subtypes) and risk of CRC (and its subsite), and to better characterize the shape of the association.
Several limitations also merit consideration. First, as a meta-analysis of observational studies, problems with confounding factors that could be inherent in the selected studies were not addressed. Second, nondifferential misclassification of exposure may have occurred because most original studies only made a single assessment of diet intake with food frequency questionnaires at baseline, leading to an attenuation of the association. Third, given the multiple stratified analyses performed, some significant findings may have occurred by chance and, therefore, should be interpreted with caution. Fourth, publication bias resulting from a tendency to publish only positive results is also a consideration. However, little evidence of such bias was found.
In summary, findings from this meta-analysis of prospective studies suggest no overall association between n-3 PUFAs intake and risk of CRC. However, our results suggest that the benefits of n-3 PUFAs on CRC might differ by subsite within colon cancer, and be restricted to initiation or early stage of CRC. Future prospective studies are needed to confirm these observations.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T (2012) American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 62:30–67
Platz EA, Willett WC, Colditz GA, Rimm EB, Spiegelman D, Giovannucci E (2000) Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control 11:579–588
Wu S, Feng B, Li K, Zhu X, Liang S, Liu X, Han S, Wang B, Wu K, Miao D, Liang J, Fan D (2012) Fish consumption and colorectal cancer risk in humans: a systematic review and meta-analysis. Am J Med 125(551–559):e555
Geelen A, Schouten JM, Kamphuis C, Stam BE, Burema J, Renkema JM, Bakker EJ, van’t Veer P, Kampman E (2007) Fish consumption, n-3 fatty acids, and colorectal cancer: a meta-analysis of prospective cohort studies. Am J Epidemiol 166:1116–1125
Mollard RC, Gillam ME, Wood TM, Taylor CG, Weiler HA (2005) (n-3) fatty acids reduce the release of prostaglandin E2 from bone but do not affect bone mass in obese (fa/fa) and lean Zucker rats. J Nutr 135:499–504
Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, Willett WC, Hu FB (2004) Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr 134:1806–1811
Ebrahimi M, Ghayour-Mobarhan M, Rezaiean S, Hoseini M, Parizade SM, Farhoudi F, Hosseininezhad SJ, Tavallaei S, Vejdani A, Azimi-Nezhad M, Shakeri MT, Rad MA, Mobarra N, Kazemi-Bajestani SM, Ferns GA (2009) Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol 64:321–327
Micallef MA, Garg ML (2009) Anti-inflammatory and cardioprotective effects of n-3 polyunsaturated fatty acids and plant sterols in hyperlipidemic individuals. Atherosclerosis 204:476–482
Baniyash M (2006) Chronic inflammation, immunosuppression and cancer: new insights and outlook. Semin Cancer Biol 16:80–88
Vidal-Vanaclocha F (2009) Inflammation in the molecular pathogenesis of cancer and atherosclerosis. Reumatol Clin 5(Suppl 1):40–43
Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A (2004) Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr 79:935–945
Shen XJ, Zhou JD, Dong JY, Ding WQ, Wu JC (2012) Dietary intake of n-3 fatty acids and colorectal cancer risk: a meta-analysis of data from 489 000 individuals. Br J Nutr 108:1550–1556
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Butler LM, Wang R, Koh WP, Stern MC, Yuan JM, Yu MC (2009) Marine n-3 and saturated fatty acids in relation to risk of colorectal cancer in Singapore Chinese: a prospective study. Int J Cancer 124:678–686
Sasazuki S, Inoue M, Iwasaki M, Sawada N, Shimazu T, Yamaji T, Takachi R, Tsugane S (2011) Intake of n-3 and n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int J Cancer 129:1718–1729
Terry P, Bergkvist L, Holmberg L, Wolk A (2001) No association between fat and fatty acids intake and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 10:913–914
Kantor ED, Lampe JW, Peters U, Vaughan TL, White E (2014) Long-chain omega-3 polyunsaturated fatty acid intake and risk of colorectal cancer. Nutr Cancer 66:716–727
Hall MN, Chavarro JE, Lee IM, Willett WC, Ma J (2008) A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev 17:1136–1143
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose–response data, with applications to meta-analysis. Am J Epidemiol 135:1301–1309
Orsini N, Bellocco R, Greenland S (2006) Generalized least squares for trend estimation of summarized dose–response data. Stata J 6:40–57
Lin J, Zhang SM, Cook NR, Lee IM, Buring JE (2004) Dietary fat and fatty acids and risk of colorectal cancer in women. Am J Epidemiol 160:1011–1022
Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D (2012) Meta-analysis for linear and nonlinear dose–response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175:66–73
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Kobayashi M, Tsubono Y, Otani T, Hanaoka T, Sobue T, Tsugane S (2004) Fish, long-chain n-3 polyunsaturated fatty acids, and risk of colorectal cancer in middle-aged Japanese: the JPHC study. Nutr Cancer 49:32–40
Koh WP, Yuan JM, van den Berg D, Lee HP, Yu MC (2004) Interaction between cyclooxygenase-2 gene polymorphism and dietary n-6 polyunsaturated fatty acids on colon cancer risk: the Singapore Chinese Health Study. Br J Cancer 90:1760–1764
Bostick RM, Potter JD, Kushi LH, Sellers TA, Steinmetz KA, McKenzie DR, Gapstur SM, Folsom AR (1994) Sugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States). Cancer Causes Control 5:38–52
Daniel CR, McCullough ML, Patel RC, Jacobs EJ, Flanders WD, Thun MJ, Calle EE (2009) Dietary intake of omega-6 and omega-3 fatty acids and risk of colorectal cancer in a prospective cohort of U.S. men and women. Cancer Epidemiol Biomarkers Prev 18:516–525
Key TJ, Appleby PN, Masset G, Brunner EJ, Cade JE, Greenwood DC, Stephen AM, Kuh D, Bhaniani A, Powell N, Khaw KT (2012) Vitamins, minerals, essential fatty acids and colorectal cancer risk in the United Kingdom Dietary Cohort Consortium. Int J Cancer 131:E320–E325
Murff HJ, Shu XO, Li H, Dai Q, Kallianpur A, Yang G, Cai H, Wen W, Gao YT, Zheng W (2009) A prospective study of dietary polyunsaturated fatty acids and colorectal cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev 18:2283–2291
Oba S, Shimizu N, Nagata C, Shimizu H, Kametani M, Takeyama N, Ohnuma T, Matsushita S (2006) The relationship between the consumption of meat, fat, and coffee and the risk of colon cancer: a prospective study in Japan. Cancer Lett 244:260–267
Pietinen P, Malila N, Virtanen M, Hartman TJ, Tangrea JA, Albanes D, Virtamo J (1999) Diet and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control 10:387–396
Song M, Chan AT, Fuchs CS, Ogino S, Hu FB, Mozaffarian D, Ma J, Willett WC, Giovannucci EL, Wu K (2014) Dietary intake of fish, omega-3 and omega-6 fatty acids and risk of colorectal cancer: a prospective study in U.S. men and women. Int J Cancer 135:2413–2423
Griffini P, Fehres O, Klieverik L, Vogels IM, Tigchelaar W, Smorenburg SM, Van Noorden CJ (1998) Dietary omega-3 polyunsaturated fatty acids promote colon carcinoma metastasis in rat liver. Cancer Res 58:3312–3319
Knekt P, Jarvinen R, Dich J, Hakulinen T (1999) Risk of colorectal and other gastro-intestinal cancers after exposure to nitrate, nitrite and N-nitroso compounds: a follow-up study. Int J Cancer 80:852–856
Loh YH, Jakszyn P, Luben RN, Mulligan AA, Mitrou PN, Khaw KT (2011) N-Nitroso compounds and cancer incidence: the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Study. Am J Clin Nutr 93:1053–1061
Macfarlane GT, Gibson GR, Cummings JH (1992) Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol 72:57–64
Povey AC, Hall CN, Badawi AF, Cooper DP, O’Connor PJ (2000) Elevated levels of the pro-carcinogenic adduct, O(6)-methylguanine, in normal DNA from the cancer prone regions of the large bowel. Gut 47:362–365
Reddy BS, Burill C, Rigotty J (1991) Effect of diets high in omega-3 and omega-6 fatty acids on initiation and post initiation stages of colon carcinogenesis. Cancer Res 51:487–491
Murff HJ, Shrubsole MJ, Cai Q, Smalley WE, Dai Q, Milne GL, Ness RM, Zheng W (2012) Dietary intake of PUFAs and colorectal polyp risk. Am J Clin Nutr 95:703–712
Pot GK, Geelen A, van Heijningen EM, Siezen CL, van Kranen HJ, Kampman E (2008) Opposing associations of serum n-3 and n-6 polyunsaturated fatty acids with colorectal adenoma risk: an endoscopy-based case–control study. Int J Cancer 123:1974–1977
Oh K, Willett WC, Fuchs CS, Giovannucci E (2005) Dietary marine n-3 fatty acids in relation to risk of distal colorectal adenoma in women. Cancer Epidemiol Biomarkers Prev 14:835–841
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
10552_2014_492_MOESM2_ESM.tif
Supplementary figure 2. Relative risk (solid line) with 95% confidence interval (long dashed lines) for the relationship between total n-3 polyunsaturated fatty acids intake and risk of colorectal cancer in men and women separately in a restricted cubic spline random-effects meta-analysis. Relative risk (solid line) with 95 % confidence interval (long dashed lines) for the relationship between total n-3 polyunsaturated fatty acids intake and risk of colorectal cancer in men and women separately in a restricted cubic spline random-effects meta-analysis. (TIFF 123 kb)
Rights and permissions
About this article
Cite this article
Chen, GC., Qin, LQ., Lu, DB. et al. N-3 polyunsaturated fatty acids intake and risk of colorectal cancer: meta-analysis of prospective studies. Cancer Causes Control 26, 133–141 (2015). https://doi.org/10.1007/s10552-014-0492-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-014-0492-1