Abstract
Dietary intake of one-carbon nutrients (methyl donors) and germline variants in the one-carbon metabolism genes may influence global DNA methylation level and methylation in promoter CpG islands. In this study, we evaluated the relationship between single nucleotide polymorphisms (SNPs) in the one-carbon metabolism pathway and DNA methylation status in colorectal cancer. Utilizing 182 colorectal cancers cases in two prospective cohort studies, we determined the CpG island methylator phenotype (CIMP) status on eight CIMP-specific promoters and measured LINE-1 methylation level that correlates well with genome-wide DNA methylation level. We genotyped 23 nonsynonymous SNPs in the one-carbon metabolism genes using buffy coat DNA. Most of the 23 SNPs in the one-carbon metabolism pathway were not significantly associated with CIMP-high status (≥6/8 methylated promoters). However, the MTHFR 429 Ala/Ala variant (rs1801131) and the TCN2 259 Arg/Arg variant (rs1801198) were associated with CIMP-high status (MTHFR 429 multivariate odds ratio (MV OR) = 7.56; 95% confidence interval (CI), 1.32–43.3; p trend = 0.10; TCN2 259 Arg/Arg variant MV OR = 3.82; 95% CI, 1.02–14.4; p trend = 0.06). The one-carbon metabolism genotypes were not significantly associated with LINE-1 methylation, although there were modest differences in mean LINE-1 methylation levels between certain genotypes. Collectively, these exploratory data provide suggestive evidence for the association of MTHFR 429 Ala/Ala and TCN2 259 Arg/Arg and CIMP status in colorectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetic and epigenetic alterations are important in carcinogenesis [1, 2]. Genome-wide DNA hypomethylation involving repetitive DNA elements such as LINE-1 (long interspersed nucleotide element-1) is considered to play an important role in genomic instability by the reactivation of transposable DNA sequences [3, 4], leading to colorectal carcinogenesis [5–9]. In contrast, aberrant promoter CpG island methylation may also contribute to colorectal cancer development by silencing tumor suppressor genes [1]. The CpG island methylator phenotype (CIMP) is characterized by widespread promoter CpG island methylation [10, 11], and inversely associated with LINE-1 hypomethylation in colorectal cancer [12]. CIMP-high colorectal cancer shows a distinctive profile, including associations with female gender, proximal tumor location, poor differentiation, microsatellite instability-high (MSI-high), and BRAF mutation [13–17].
Genetic factors such as germline single nucleotide polymorphisms (SNPs), along with dietary intake of one-carbon nutrients such as folate and methionine [18, 19], likely influence cellular one-carbon metabolism and methyl-donor status [20, 21]. Recent studies have shown the potential relationship between germline variants in methyl-group metabolism genes and promoter CpG island methylation in colorectal tumors [22, 23].
We have previously shown that polymorphisms in the B12-methionine-related pathway have been associated with colorectal cancer [24] and colorectal adenoma [25]. In this study, we examined the relationship between 23 germline nonsynonymous SNPs (nsSNPs) in 12 one-carbon metabolism genes and DNA methylation status (CIMP status and LINE-1 methylation) in colorectal cancer.
Materials and methods
Study population
The Nurses’ Health Study (NHS) is an ongoing prospective study of 121,700 US female registered nurses. Details of the design and follow-up of this cohort have been previously described [26]. Briefly, at enrollment in 1976, the participants, who were aged 30–55, completed a questionnaire providing information on risk factors for cancer and cardiovascular disease. Exposure and disease information are updated biennially. From 1989 to 1990, blood samples were collected from 32,826 of the NHS participants. After blood collection through June 2000, 197 incident cases of colorectal cancer were confirmed through medical records or death reports, of which 190 cases were successfully genotyped.
The Health Professionals Follow-up Study (HPFS) began in 1986 when 51,529 US male health professionals (dentists, optometrists, osteopaths, podiatrists, pharmacists, and veterinarians), aged 40–75, responded to a mailed questionnaire. These men provided baseline information on age, marital status, height, weight, ancestry, medications, smoking history, medical history, physical activity, and diet. Information on exposure and medical history is updated every 2 years. Blood samples were collected from 18,225 of the HPFS participants between 1993 and 1995; 168 incident cases of colorectal cancer were identified between the date of blood draw and January 2002.
On each biennial follow-up questionnaire, participants were asked whether they had had a diagnosis of colorectal cancer during the prior 2 years. When a participant reported a diagnosis of colorectal cancer, we asked for permission to obtain hospital records and pathology reports. For persistent nonresponders, we searched for National Death Index to identify potential colorectal cancer-related deaths. We identified more than 96% of incident colorectal cancers by these methods. Study physicians, blinded to exposure data, reviewed all medical records related to colorectal cancer, classifying disease stage according to the TNM (tumor-node-metastasis) classification.
In each cohort, individuals who were alive and free of diagnosed cancer at the time of case ascertainment were selected as controls and were matched to cases on year of birth and year and month of blood draw, as previously described [26, 27].
Previous studies that were based on the NHS and HPFS have described baseline characteristics of cohort participants and incident colorectal cancer cases and confirmed that our colorectal cancer cases were representative as a population-based sample [28]. We collected paraffin-embedded tissue blocks from hospitals where cohort participants with colorectal cancers had undergone resections of primary tumors. Specimens were selected based on availability of germline SNP analysis data and tumor analysis data at the time of this study. Table 1 indicates that there was no substantial bias in terms of clinical and pathologic features. Tumor content was more than 70% for all cases. All cases were confirmed by a single pathologist (S.O.) to be colorectal cancer, but not stromal tumor, carcinoid, lymphoma, or metastatic tumor from another organ site. Based on availability of tumor tissue specimens, a total of 182 colorectal cancer cases (100 from the HPFS and 82 from the NHS) were included. Overall, these cases were similar in epidemiologic features to the total number of samples in the NHS and HPFS with blood samples and similar in pathologic features to the total number of cases with CIMP and LINE-1 data. Cases were previously characterized for status of CIMP [29], LINE-1 methylation [12], and one-carbon metabolism germline SNPs [24, 25]. However, no study has been performed to correlate these 23 germline nonsynonymous SNPs in the one-carbon metabolism pathway with CIMP status and LINE-1 methylation in tumors. Blood collection, germline SNP analyses, and tumor tissue analyses were approved by the Institutional Review Boards of the Harvard School of Public Health, Dana-Farber Cancer Institute, and Brigham and Women’s Hospital.
Genotyping methods
Genotyping was performed at the Dana-Farber/Harvard Cancer Center High-Throughput Polymorphism Core. DNA was extracted from 50-μl buffy coat fractions diluted with 150 μl of PBS by the Qiagen QIAamp Blood Kit (Qiagen, Chatsworth, CA) spin protocol. The genotypes of the one-carbon polymorphisms were determined by measuring end-point fluorescence using the 5′ nuclease assay (Taqman) on an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) [30]. The 23 nonsynonymous SNPs were identified through the NCI SNP500Cancer database (http://snp500cancer.nci.nih.gov), the dbSNP database (http://www.ncbi.nlm.nih.gov/SNP), and the International HapMap Project database (http://www.hapmap.org). The SNPs studied all resulted in amino acid changes and therefore are potentially functional. The FOLH1 His475Tyr SNP was not referenced in the above-mentioned databases but was investigated in relation to serum folate levels [31]. Our analysis included the 23 known nonsynonymous SNPs in 12 genes [24, 25] that could be evaluated by the Taqman assay (SHMT was excluded from this analysis due to lower overlap of successfully genotyped blood samples with CIMP and LINE-1 data from somatic tissue).
Quality control was ensured by including a random 10% of the samples in the 96-well plates as duplicates. These served as internal controls to validate the genotyping methods; there was 100% concordance. Laboratory personnel were blinded to the status (case, control, or quality control) of samples. The median genotyping success for the 23 SNPs included in this analysis was 95%.
Quantitative real-time PCR (MethyLight) for CIMP analysis
Sodium bisulfite treatment on tumor DNA and subsequent real-time PCR (MethyLight) assays were validated and performed as previously described [32]. We quantified promoter methylation in eight CIMP-specific genes (CACNA1G, CDKN2A (p16), CRABP1, IGF2, MLH1, NEUROG1, RUNX3, and SOCS1) [15, 33]. The PCR condition was initial denaturation at 95°C for 10 min followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. CIMP-high was defined as ≥6/8 methylated promoters using the 8-marker CIMP panel, CIMP-low as 1–5 methylated promoters, and CIMP-0 as 0/8 methylated promoters, according to the previously established criteria [33]. Accumulating evidence indicate that CIMP-high is a distinct entity with tight associations with MSI and BRAF mutation. Although CIMP-low is associated with KRAS mutation [32, 34], the relatedness between CIMP-low and CIMP-0 is closer to that between CIMP-low and CIMP-high [32]. Thus, because of limited power, we combined CIMP-low and CIMP-0 in this study.
Pyrosequencing to measure LINE-1 methylation
In order to accurately quantify relatively high LINE-1 methylation levels, we utilized Pyrosequencing technology as previously described [35, 36]. The amount of C relative to the sum of the amounts of C and T at each CpG site was calculated as percentage. The average of the relative amounts of C in the four CpG sites was used as overall LINE-1 methylation level in a given tumor. LINE-1 methylation level measured by Pyrosequencing has been shown to correlate well with overall 5-methylcytosine level (i.e., global DNA methylation level) in tumor cells [9, 35].
Statistical analysis
We evaluated the relationship between each of the 23 SNPs in the one-carbon metabolism pathway and DNA methylation status (CIMP status or LINE-1 methylation) in colorectal cancer cases (case–case analysis). We used the codominant genetic model (when appropriate) as well as the dominant genetic model, comparing variant carriers with the referent homozygous wild type. The genotype distributions for the SNPs were evaluated for agreement with Hardy–Weinberg equilibrium (HWE) by the trend test. We used unconditional logistic regression for the analyses to compute odds ratios (ORs) with 95% confidence intervals (CIs). The risk for CIMP-high was evaluated using logistic regression with an ordinal outcome variable, modeled as a three-level categorical model: CIMP-high, CIMP-low, and CIMP-0. Due to sample size constraints, the results from the collapsed two-level categorical variable: CIMP-high and CIMP-low/0 are presented. The association between LINE-1 levels (a continuous variable) and the SNPs was tested by the Wilcoxon rank sum method. Multivariate unconditional logistic regression (for CIMP data) and multivariate linear regression (for LINE-1 data) analyses were adjusted for age, sex, family history of colon cancer, pack years smoked, body mass index (BMI), postmenopausal hormone use, aspirin intake, physical activity, alcohol intake, total folate consumption, and red meat consumption. p values were obtained from the tests for linear trend of log-ORs were calculated using an ordered categorical variable by assigning scores to the genotypes: 0 (no variant allele), one (carrying one variant allele), and two (carrying two variant alleles). Similar results were obtained with two-degree of freedom likelihood ratio test (2-df LRT) comparing models with and without the genotype variable. All statistical tests were two-sided. In our previous study of one-carbon metabolism SNPs and colorectal adenoma, we evaluated linkage disequilibrium of these 23 SNPs. The conservative Bonferroni-corrected p value for the independent tests is alpha/20 markers = 0.003. All statistical analyses were performed with SAS (version 9.1; SAS Institute, Cary, NC).
Results
The clinical characteristics of the 182 colorectal cancer cases are presented in Table 1. CIMP-high (defined as the presence of ≥6/8 methylated promoters) was detected in 31 (17%) of the 182 colorectal cancers. LINE-1 methylation levels in the 172 colorectal cancers were distributed approximately normally (mean 61.81, standard deviation 10.11, median 62.49, interquartile range 12.38).
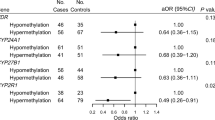
We compared the distribution of the genotypes (Table 2) in the CIMP-high to a reference group of CIMP-low/0 (with ≤5/8 methylated promoters). Modest differences in genotype distributions were noted for TCN2 (transcobalamin 2) Pro259Arg (dbSNP ID: rs1801198; p trend = 0.06). In multivariate logistic regression analysis, the MTHFR (methylenetetrahydrofolate reductase) 429 Ala/Ala variant was associated with an increased risk of CIMP-high (dbSNP ID: rs1801131; OR = 7.56; 95% CI, 1.32–43.3; p trend = 0.11) (Table 3). In contrast, the MTHFR Ala222Val (dbSNP ID: rs1801133) genotypes were not significantly associated with CIMP-high. In the B12-related cycle of the one-carbon metabolism pathway, the TCN2 259 Arg/Arg variant (OR = 3.82, 95% CI: 1.02–14.4; p value = 0.06) was associated with CIMP-high. All of the other SNPs in the one-carbon metabolism pathway were not significantly associated with CIMP-high (Table 3). Similar point estimates were obtained comparing CIMP-high to CIMP-low (excluding CIMP-0) in the age-adjusted model (data not shown). MTHFR 429 Ala/Ala was significantly associated with CIMP-high (OR = 7.39; 95% CI, 1.2–45.1, p trend = 0.03). The association for TCN2 259 Arg/Arg was slightly attenuated with OR = 2.91 (95% CI, 0.85–9.92, p trend = 0.06). Only a few cases with CIMP-high had distal or rectal tumors located in the rectum; therefore, we were unable to perform stratified analysis by location. However, these associations were not statistically significant after correcting for multiple comparisons.
We next measured LINE-1 methylation, which has been correlated with global DNA methylation level [35]. There was no significant difference in overall median LINE-1 methylation levels and the 23 nonsynonymous SNPs examined (Table 4) or by stratified analysis by tumor location (colon compared to rectal; data not shown). However, there were some differences in mean LINE-1 methylation levels between certain genotypes (i.e., FTHFD 254).
Discussion
We conducted this study to examine the relationship between germline polymorphisms in the one-carbon metabolism genes and DNA methylation status (CpG island methylator phenotype (CIMP) and LINE-1 methylation level) in colorectal cancer. We used quantitative PCR assays (MethyLight) [37] to determine the degree of DNA methylation, which is essential to reproducibly differentiate high-level from low-level methylation [32]. With the use of the CIMP-specific panel of eight promoters [15, 33], we were able to accurately identify CIMP-high in colorectal cancer. We have found that the MTHFR 429 Ala/Ala variant and the TCN2 259 Arg/Arg variant were associated with CIMP-high status in colorectal cancer. Our data support the possible link between these germline genetic variants and somatic promoter CpG island methylation in colorectal cancer.
The combination of dietary factors and genetic and epigenetic variation contributes to colorectal cancer risk. Intake of folate, especially among alcohol consumers, is protective against the risk of colorectal cancer [20]. Diet, lifestyle, and environment [17, 38] contribute to carcinogenesis by inducing both genetic and epigenetic changes that in combination result in the disruption of key cellular process leading to neoplastic transformation. Folate from diet is directly linked to DNA methylation via the one-carbon metabolism pathway, where S-adenosylmethionine (SAM) [39] is the universal methyl donor for several biological methylation reactions and for de novo deoxynucleoside triphosphate synthesis. The reduced availability of methyltetrahydrofolate (methyl-THF), the main circulating form of folate, decreases the biosynthesis of SAM, thus limiting the availability of methyl groups for methylation reactions [40].
Thus, dietary folate consumption may modify the association between DNA methylation and colorectal cancer risk [23, 28, 41–44]. Examining epigenetic changes in colorectal cancer in relation to genetic and dietary factors is important because epigenetic heterogeneity that exists among colorectal cancers may be caused by genetic and environmental factors. However, few epidemiologic studies have investigated the role of epigenetic changes induced by dietary and environmental exposures [17]. In the one-carbon metabolism pathway, the MTHFR Ala222Val (677C > T) polymorphism (NM_005957.3) has been associated with decreased enzyme activity, folate status and global hypomethylation detected in lymphocytes [40]. The MTHFR 429 (1298A > C) polymorphisms is also associated with decreased enzymatic activity and hyperhomocysteinemia [45]. Intracellular folate levels (5,10-methylenetetrahydrofolate and tetrahydrofolate) have also been associated with promoter methylation of MLH1, TIMP3, ARF (CDKN2A/p14), and the MTHFR SNPs [46]. However, a recent large population-based study did not find an association among folate, vitamin B6, vitamin B12, methionine, and CIMP-high [17]. Furthermore, studies have not found an association between the MTHFR haplotypes and promoter methylation status in proximal colon cancer [47] or colorectal adenoma [48]. Thus, other risk factors may be indirectly related to CIMP-high in colorectal cancer.
Analysis of genetic and epigenetic alterations, such as DNA hypermethylation and global hypomethylation is important in cancer research [1, 49–62]. We have previously described CIMP status and LINE-1 methylation in this large population-based sample of colorectal cancer [12, 29]. In this exploratory study, we evaluated the relationship of CIMP status and LINE-1 methylation with 23 nonsynonymous polymorphisms (SNPs) in 12 genes in the one-carbon pathway. Most of the SNPs in the one-carbon metabolism pathway were not associated with CIMP-high or LINE-1 methylation levels in colorectal cancer, although we may be underpowered to evaluate these associations given our sample size and due to multiple comparisons. We have shown an association between the MTHFR 429 Ala/Ala variant and CIMP-high in colorectal cancer, which was modestly stronger than the association observed in a larger population-based study of colon cancer evaluating SNPs in eight one-carbon metabolism genes [23]. In addition to sample size differences or chance, this difference in the magnitude of association may be in part due to differences in the CIMP markers and criteria in these two studies; Curtin et al. [23] used the classic CIMP marker panel (MINT1, MINT2, MINT31, CDKN2A, and MLH1), which is different from our 8-marker CIMP panel [15, 33]. Another recent large study suggests that the MTHFR 222 Val/Val variant may be associated with MSI cases of colorectal cancer [63]. Although CIMP status was not determined in this study, these results collectively support the relationship between the MTHFR SNPs and CIMP in colorectal cancer.
In the B12-related cycle of the one-carbon metabolism pathway, the TCN2 259 Arg/Arg variant had an association with CIMP-high. However, Curtin et al. [23] reported a reduced risk of colon cancer for the variant carriers of TCN2. In the Nurses’ Health Study (NHS), the TCN2 259 Pro/Arg + Arg/Arg genotypes were associated with increased risk of colorectal adenoma [25] but not cancer [24]. Additional studies are necessary to examine the relation between the TCN2 Pro259Arg polymorphism and DNA methylation status in colorectal cancer. The relationship of germline genetic variation and somatic methylation is an emerging area of investigation [22, 23, 47, 48]. This exploratory population-based study provides evidence for the possible relationship between germline nonsynonymous SNPs in the one-carbon metabolism pathway and DNA methylation in tumors and suggests the need for further studies on the MTHFR 429 polymorphism, the methionine-cycle and B12-related genes, and the FTHFD gene. We were limited in this analysis by the overlap of genotype data available on incident colorectal cancer cases and methylation data from colorectal cancer tissue. In addition to dietary methyl status, other risk factors, such as smoking [17, 38], may be indirectly related to CIMP-high. The association of DNA methylation and lifestyle factors [57, 64] remains an important area of cancer research and the current findings should be evaluated in larger studies with prospective data on risk factors for cancer.
In summary, MTHFR 429 Ala/Ala and TCN2 259 Arg/Arg in the one-carbon metabolism pathway are associated with CIMP-high in colorectal cancer, although these findings may be due to chance. Further studies are necessary to elucidate the exact mechanisms of this association.
Abbreviations
- BHMT:
-
Betaine–homocysteine methyltransferase
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CIMP:
-
CpG island methylator phenotype
- HPFS:
-
Health Professionals Follow-up Study
- LINE-1:
-
Long interspersed nucleotide element-1
- MSI:
-
Microsatellite instability
- MTHFR:
-
Methylenetetrahydrofolate reductase
- MTRR:
-
5-Methyltetrahydrofolate–homocysteine methyltransferase reductase (methionine synthase reductase)
- NHS:
-
Nurses’ Health Study
- nsSNP:
-
Nonsynonymous single nucleotide polymorphism
- OR:
-
Odds ratio
- SNP:
-
Single nucleotide polymorphism
- TCN2:
-
Transcobalamin 2
References
Esteller M (2008) Epigenetics in cancer. N Engl J Med 358(11):1148–1159
Feinberg AP (2008) Epigenetics at the epicenter of modern medicine. Jama 299(11):1345–1350
Ji W, Hernandez R, Zhang XY, Qu GZ, Frady A, Varela M et al (1997) DNA demethylation and pericentromeric rearrangements of chromosome 1. Mutat Res 379(1):33–41
Gaudet F, Hodgson JG, Eden A, Jackson-Grusby L, Dausman J, Gray JW et al (2003) Induction of tumors in mice by genomic hypomethylation. Science 300(5618):489–492
Yamada Y, Jackson-Grusby L, Linhart H, Meissner A, Eden A, Lin H et al (2005) Opposing effects of DNA hypomethylation on intestinal and liver carcinogenesis. Proc Natl Acad Sci USA 102(38):13580–13585
Rodriguez J, Frigola J, Vendrell E, Risques RA, Fraga MF, Morales C et al (2006) Chromosomal instability correlates with genome-wide DNA demethylation in human primary colorectal cancers. Cancer Res 66(17):8462–9468
Suzuki K, Suzuki I, Leodolter A, Alonso S, Horiuchi S, Yamashita K et al (2006) Global DNA demethylation in gastrointestinal cancer is age dependent and precedes genomic damage. Cancer Cell 9(3):199–207
Karpf AR, Matsui S (2005) Genetic disruption of cytosine DNA methyltransferase enzymes induces chromosomal instability in human cancer cells. Cancer Res 65(19):8635–8639
Estecio MR, Gharibyan V, Shen L, Ibrahim AE, Doshi K, He R et al (2007) LINE-1 hypomethylation in cancer is highly variable and inversely correlated with microsatellite instability. PLoS ONE 2(5):e399
Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP (1999) CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA 96(15):8681–8686
Shen L, Toyota M, Kondo Y, Lin E, Zhang L, Guo Y et al (2007) Integrated genetic and epigenetic analysis identifies three different subclasses of colon cancer. Proc Natl Acad Sci USA 104(47):18654–18659
Ogino S, Kawasaki T, Nosho K, Ohnishi M, Suemoto Y, Kirkner GJ et al (2008) LINE-1 hypomethylation is inversely associated with microsatellite instability and CpG methylator phenotype (CIMP) in colorectal cancer. Int J Cancer 122:2767–2773
Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD et al (2004) BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut 53(8):1137–1144
Samowitz W, Albertsen H, Herrick J, Levin TR, Sweeney C, Murtaugh MA et al (2005) Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology 129(3):837–845
Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA et al (2006) CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet 38(7):787–793
Kawasaki T, Nosho K, Ohnishi M, Suemoto Y, Kirkner GJ, Fuchs CS et al (2007) IGFBP3 promoter methylation in colorectal cancer: relationship with microsatellite instability, CpG island methylator phenotype, and p53. Neoplasia 9(12):1091–1098
Slattery ML, Curtin K, Sweeney C, Levin TR, Potter J, Wolff RK et al (2007) Diet and lifestyle factor associations with CpG island methylator phenotype and BRAF mutations in colon cancer. Int J Cancer 120(3):656–663
Flood A, Caprario L, Chaterjee N, Lacey JV Jr, Schairer C, Schatzkin A (2002) Folate, methionine, alcohol, and colorectal cancer in a prospective study of women in the United States. Cancer Causes Control 13(6):551–561
Boyapati SM, Bostick RM, McGlynn KA, Fina MF, Roufail WM, Geisinger KR et al (2004) Folate intake, MTHFR C677T polymorphism, alcohol consumption, and risk for sporadic colorectal adenoma (United States). Cancer Causes Control 15(5):493–501
Giovannucci E (2004) Alcohol, one-carbon metabolism, and colorectal cancer: recent insights from molecular studies. J Nutr 134(9):2475S–2481S
Chen J, Kyte C, Valcin M, Chan W, Wetmur JG, Selhub J et al (2004) Polymorphisms in the one-carbon metabolic pathway, plasma folate levels and colorectal cancer in a prospective study. Int J Cancer 110(4):617–620
Murrell A, Heeson S, Cooper WN, Douglas E, Apostolidou S, Moore GE et al (2004) An association between variants in the IGF2 gene and Beckwith–Wiedemann syndrome: interaction between genotype and epigenotype. Hum Mol Genet 13(2):247–255
Curtin K, Slattery ML, Ulrich CM, Bigler J, Levin TR, Wolff RK et al (2007) Genetic polymorphisms in one-carbon metabolism: associations with CpG island methylator phenotype (CIMP) in colon cancer and the modifying effects of diet. Carcinogenesis 28(8):1672–1679
Koushik A, Kraft P, Fuchs CS, Hankinson SE, Willett WC, Giovannucci EL et al (2006) Nonsynonymous polymorphisms in genes in the one-carbon metabolism pathway and associations with colorectal cancer. Cancer Epidemiol Biomarkers Prev 15(12):2408–2417
Hazra A, Wu K, Kraft P, Fuchs CS, Giovannucci EL, Hunter DJ (2007) Twenty-four non-synonymous polymorphisms in the one-carbon metabolic pathway and risk of colorectal adenoma in the Nurses’ Health Study. Carcinogenesis 28(7):1510–1519
Colditz GA, Hankinson SE (2005) The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer 5(5):388–396
Tranah GJ, Bugni J, Giovannucci E, Ma J, Fuchs C, Hines L et al (2006) O6-methylguanine-DNA methyltransferase Leu84Phe and Ile143Val polymorphisms and risk of colorectal cancer in the Nurses’ Health Study and Physicians’ Health Study (United States). Cancer Causes Control 17(5):721–731
Tranah GJ, Giovannucci E, Ma J, Fuchs C, Hunter DJ (2005) APC Asp1822Val and Gly2502Ser polymorphisms and risk of colorectal cancer and adenoma. Cancer Epidemiol Biomarkers Prev 14(4):863–870
Ogino S, Cantor M, Kawasaki T, Brahmandam M, Kirkner G, Weisenberger DJ et al (2006) CpG island methylator phenotype (CIMP) of colorectal cancer is best characterised by quantitative DNA methylation analysis and prospective cohort studies. Gut 55:1000–1006
Livak KJ (1999) Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal 14(5–6):143–149
Devlin AM, Ling EH, Peerson JM, Fernando S, Clarke R, Smith AD et al (2000) Glutamate carboxypeptidase II: a polymorphism associated with lower levels of serum folate and hyperhomocysteinemia. Hum Mol Genet 9(19):2837–2844
Ogino S, Kawasaki T, Brahmandam M, Cantor M, Kirkner GJ, Spiegelman D et al (2006) Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn 8(2):209–217
Ogino S, Kawasaki T, Kirkner GJ, Kraft P, Loda M, Fuchs CS (2007) Evaluation of markers for CpG island methylator phenotype (CIMP) in colorectal cancer by a large population-based sample. J Mol Diagn 9(3):305–314
Barault L, Charon-Barra C, Jooste V, de la Vega MF, Martin L, Roignot P et al (2008) Hypermethylator phenotype in sporadic colon cancer: study on a population-based series of 582 cases. Cancer Res 68(20):8541–8546
Yang AS, Estecio MR, Doshi K, Kondo Y, Tajara EH, Issa JP (2004) A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res 32(3):e38
Ogino S, Kawasaki T, Nosho K, Ohnishi M, Suemoto Y, Kirkner GJ et al (2008) LINE-1 hypomethylation is inversely associated with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Int J Cancer 122(12):2767–2773
Eads CA, Danenberg KD, Kawakami K, Saltz LB, Blake C, Shibata D et al (2000) MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res 28(8):E32
Liu Y, Lan Q, Siegfried JM, Luketich JD, Keohavong P (2006) Aberrant promoter methylation of p16 and MGMT genes in lung tumors from smoking and never-smoking lung cancer patients. Neoplasia 8(1):46–51
Alonso-Aperte E, Gonzalez MP, Poo-Prieto R, Varela-Moreiras G (2008) Folate status and S-adenosylmethionine/S-adenosylhomocysteine ratio in colorectal adenocarcinoma in humans. Eur J Clin Nutr 62(2):295–298
Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ et al (2002) A common mutation in the 5, 10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci USA 99(8):5606–5611
Giovannucci E, Stampfer MJ, Colditz GA, Rimm EB, Trichopoulos D, Rosner BA et al (1993) Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst 85(11):875–884
Sharp L, Little J, Brockton NT, Cotton SC, Masson LF, Haites NE et al (2008) Polymorphisms in the methylenetetrahydrofolate reductase (MTHFR) gene, intakes of folate and related B vitamins and colorectal cancer: a case–control study in a population with relatively low folate intake. Br J Nutr 99(2):379–389
Brockton NT (2006) Localized depletion: the key to colorectal cancer risk mediated by MTHFR genotype and folate? Cancer Causes Control 17(8):1005–1016
Brockton NT (2008) Systemic folate status and risk of colorectal cancer. Cancer Causes Control 19(9):1005–1007 (author reply 3)
Weisberg IS, Jacques PF, Selhub J, Bostom AG, Chen Z, Curtis Ellison R et al (2001) The 1298A → C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression and association with homocysteine. Atherosclerosis 156(2):409–415
Kawakami K, Ruszkiewicz A, Bennett G, Moore J, Watanabe G, Iacopetta B (2003) The folate pool in colorectal cancers is associated with DNA hypermethylation and with a polymorphism in methylenetetrahydrofolate reductase. Clin Cancer Res 9(16 Pt 1):5860–5865
Oyama K, Kawakami K, Maeda K, Ishiguro K, Watanabe G (2004) The association between methylenetetrahydrofolate reductase polymorphism and promoter methylation in proximal colon cancer. Anticancer Res 24(2B):649–654
van den Donk M, van Engeland M, Pellis L, Witteman BJ, Kok FJ, Keijer J et al (2007) Dietary folate intake in combination with MTHFR C677T genotype and promoter methylation of tumor suppressor and DNA repair genes in sporadic colorectal adenomas. Cancer Epidemiol Biomarkers Prev 16(2):327–333
Yu J, Yu J, Almal AA, Dhanasekaran SM, Ghosh D, Worzel WP et al (2007) Feature selection and molecular classification of cancer using genetic programming. Neoplasia 9(4):292–303
Tsareva SA, Moriggl R, Corvinus FM, Wiederanders B, Schutz A, Kovacic B et al (2007) Signal transducer and activator of transcription 3 activation promotes invasive growth of colon carcinomas through matrix metalloproteinase induction. Neoplasia 9(4):279–291
Ateeq B, Unterberger A, Szyf M, Rabbani SA (2008) Pharmacological inhibition of DNA methylation induces proinvasive and prometastatic genes in vitro and in vivo. Neoplasia 10(3):266–278
Nagasaka T, Koi M, Kloor M, Gebert J, Vilkin A, Nishida N et al (2008) Mutations in both KRAS and BRAF may contribute to the methylator phenotype in colon cancer. Gastroenterology 134(7):1950–1960
Samowitz WS (2008) Genetic and epigenetic changes in colon cancer. Exp Mol Pathol 85(1):64–67
Sawan C, Vaissiere T, Murr R, Herceg Z (2008) Epigenetic drivers and genetic passengers on the road to cancer. Mutat Res 642(1–2):1–13
Thorstensen L, Lind GE, Lovig T, Diep CB, Meling GI, Rognum TO et al (2005) Genetic and epigenetic changes of components affecting the WNT pathway in colorectal carcinomas stratified by microsatellite instability. Neoplasia 7(2):99–108
Rocken C, Neumann K, Carl-McGrath S, Lage H, Ebert MP, Dierkes J et al (2007) The gene polymorphism of the angiotensin I-converting enzyme correlates with tumor size and patient survival in colorectal cancer patients. Neoplasia 9(9):716–722
Campos AC, Molognoni F, Melo FH, Galdieri LC, Carneiro CR, D’Almeida V et al (2007) Oxidative stress modulates DNA methylation during melanocyte anchorage blockade associated with malignant transformation. Neoplasia 9(12):1111–1121
Blanco D, Vicent S, Fraga MF, Fernandez-Garcia I, Freire J, Lujambio A et al (2007) Molecular analysis of a multistep lung cancer model induced by chronic inflammation reveals epigenetic regulation of p16 and activation of the DNA damage response pathway. Neoplasia 9(10):840–852
Kuester D, Dar AA, Moskaluk CC, Krueger S, Meyer F, Hartig R et al (2007) Early involvement of death-associated protein kinase promoter hypermethylation in the carcinogenesis of Barrett’s esophageal adenocarcinoma and its association with clinical progression. Neoplasia 9(3):236–245
Litkouhi B, Kwong J, Lo CM, Smedley JG III, McClane BA, Aponte M et al (2007) Claudin-4 overexpression in epithelial ovarian cancer is associated with hypomethylation and is a potential target for modulation of tight junction barrier function using a C-terminal fragment of Clostridium perfringens enterotoxin. Neoplasia 9(4):304–314
Lim U, Flood A, Choi SW, Albanes D, Cross AJ, Schatzkin A et al (2008) Genomic methylation of leukocyte DNA in relation to colorectal adenoma among asymptomatic women. Gastroenterology 134(1):47–55
Bjornsson HT, Sigurdsson MI, Fallin MD, Irizarry RA, Aspelund T, Cui H et al (2008) Intra-individual change over time in DNA methylation with familial clustering. Jama 299(24):2877–2883
Hubner RA, Lubbe S, Chandler I, Houlston RS (2007) MTHFR C677T has differential influence on risk of MSI and MSS colorectal cancer. Hum Mol Genet 16(9):1072–1077
Anacleto C, Leopoldino AM, Rossi B, Soares FA, Lopes A, Rocha JC et al (2005) Colorectal cancer “methylator phenotype”: fact or artifact? Neoplasia 7(4):331–335
Acknowledgments
This research is supported by the National Institutes of Health Research Grants U54 CA100971, P01 CA87969, P01 CA55075, R01 CA070817, P50 CA127003, R03 CA142082, and K07 CA122826 (to S.O.); the Bennett Family Fund; and the Entertainment Industry Foundation’s National Colorectal Cancer Research Alliance. A.H. was supported in part by training grant NIH T-32 CA 09001-30. The content is solely the responsibility of the authors and does not necessarily represent the official views of NCI or NIH. Funding agencies did not have any role in the design of the study; the collection, analysis, or interpretation of the data; the decision to submit the manuscript for publication; or the writing of the manuscript. We deeply thank the Nurses’ Health Study and Health Professionals Follow-up Study cohort participants who generously agreed to provide us with biological specimens and information through responses to questionnaires; hospitals and pathology departments throughout the US for providing us with tumor tissue materials; Walter Willett, Sue Hankinson, and many other staff members who implemented and have maintained the cohort studies; Jean-Pierre Issa, Lanlan Shen, and Liying Yan for their assistance in the LINE-1 Pyrosequencing assay; and Peter Laird and Daniel Weisenberger for their assistance in the MethyLight assay.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hazra, A., Fuchs, C.S., Kawasaki, T. et al. Germline polymorphisms in the one-carbon metabolism pathway and DNA methylation in colorectal cancer. Cancer Causes Control 21, 331–345 (2010). https://doi.org/10.1007/s10552-009-9464-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-009-9464-2