Abstract
Purpose
Triple-negative breast cancer (TNBC) is an aggressive breast cancer histological type that is predictive of poor outcomes, shorter remission periods and reduced survival. TNBC is treated with surgery and neo/adjuvant chemotherapy, with evidence of association between longer periods from surgery to adjuvant chemotherapy (time to chemotherapy, TTC) and poorer survival outcomes. This study investigated regional differences in TTC period between regions and ethnic groups to evaluate equity of care in the English TNBC population. Time from neoadjuvant chemotherapy to surgery (time to surgery, TTS) was also compared between groups.
Methods
This retrospective cohort study compared TTC and TTS periods in TNBC patients in England over a two-year period. TTC and TTS were compared by English region and ethnicity, testing for significant differences in treatment pathway timing by these demographics.
Results
1347 TNBC patients were included in the study. Significant regional differences in TTC were observed, with the longest median period of 50 days (IQR 36, 83) in the Midlands compared to 38 days (IQR 27, 55) in the North West (p < 0.001). No significant differences in TTS were observed between regions. Ethnicity was not significantly associated with timeliness of neo/adjuvant chemotherapy initiation (p > 0.05).
Conclusion
These findings suggest regional differences in TTC for patients treated with surgery and chemotherapy for TNBC. Given evidence of increased mortality risk as the TTC period increases, the causes of regional disparities warrant further investigation. This study can inform targets for improvement in the delivery of adjuvant chemotherapy in cancer treatment centres in England.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer in women and the leading cause of cancer death worldwide [1], accounting for almost one third of all new cancer diagnoses in women in 2022 in the US [2] and 25.5% in the UK [3]. Triple negative breast cancer (TNBC) is an aggressive subtype of BC, accounting for approximately 15% of BC diagnoses [4]. TNBC patients typically have a poorer prognosis compared to other subtypes, with shorter remission periods and more aggressive malignancy [5, 6]. Because of this, prompt initiation of neo/adjuvant treatment before and after surgery is important in TNBC patients. Chemotherapy combined with surgery is the main treatment for this patient group [7, 8], due to the lack of actionable receptors for targeted or hormonal therapies in TNBC. Immunotherapy and targeted therapies are now given in neo/adjuvant settings; however, chemotherapy remains an important backbone of treatment for TNBC.
TTC (time to chemotherapy) is the time period between surgery and the first administration of adjuvant chemotherapy. Longer TTC and delayed initiation of adjuvant chemotherapy has been associated with reduced overall survival (OS) and breast cancer-specific survival (BCSS) in TNBC [9] and patients with other hormone status [10, 11]. It is therefore important for patients in this high-risk group to receive timely administration of chemotherapy to maximise treatment benefit. Studies investigating the impact of time from neoadjuvant chemotherapy (NACT) to surgery (time to surgery, TTS) also suggest equivalent patient outcomes when surgery is performed with 8 weeks of completion of NACT; however, survival outcomes worsen when the TTS period exceeds 8 weeks [12].
Previous studies have highlighted variation in TTC between different ethnic groups worldwide. Non-Hispanic Black and Hispanic women were found to be significantly more likely to have a TTC of 91 days or more, compared to White women [13], and a greater proportion of indigenous Māori women in New Zealand, (37.3%), experienced delayed adjuvant chemotherapy compared to European women (30.5%) [14]. These studies have observed statistically significant differences in time to adjuvant chemotherapy between ethnic groups, raising questions on disparity in the highly multicultural and variable context of the patient population in England.
In the present study, the primary aim was to assess differences in neo/adjuvant treatment timing relative to surgery in TNBC patients, comparing TTC and TTS between the seven commissioning regions of England and by patient ethnicity. Given the already poor survival outcomes experienced by this patient group [5, 6], and the potential impact of prolonged TTC on survival outcomes [9] [11] [15], investigating this issue in the English TNBC population was of importance to guide future research and practice improvements. This analysis evaluated the effect of ethnic, regional and socioeconomic background on treatment pathways in TNBC patients in England treated in the 2014–2015 study period, assessing parity of care of with respect to the clinically significant prognostic factor of TTC (see Supplementary Table 1).
Methods
Data source
Data were requested from the National Disease Registration Service (NDRS) for early-stage (stage 2/3) BC patients treated with systemic anti-cancer therapy between January 1st 2014, and December 31st 2015. Pseudonymised patient-level datasets were provided by the National Cancer Registration and Analysis Service (NCRAS). Key variables included sex, date of birth, ethnicity, index of multiple deprivation (derived from postcode of residence at diagnosis), date and type of surgery (curative/non-curative), stage at diagnosis, and hormone status (oestrogen (ER), progesterone (PR) and human epidermal growth factor receptor-2 (HER-2)). Follow up status was provided from Electronic Health Record systems, with date of death records linked to Office for National Statistics data.
The Systemic Anti-Cancer Therapy (SACT) dataset is a population-based record of systemic treatments given for cancer given in NHS centres in England. Key data fields include specific SACT drug, dose and date of administration, organisation code of the treating centre, and patient height and weight. Patient records were linked using common pseudonymised identifiers.
Study population
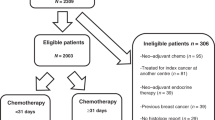
This retrospective study cohort included all patients with early-stage (stage 2 or 3) TNBC (ER-negative, PR-negative and HER2-negative), with records of surgery and chemotherapy treatment. Patients listed with diagnosis listed as stage 2A or 2B were combined as stage 2, and 3A, 3B and 3C were grouped as stage 3. Adult patients ≥ 18 years of age were eligible for inclusion.
Defining TNBC status
Patients were assigned hormone status based on available receptor status data. Patients were labelled as TNBC when ER, HER2 and PR status were recorded as negative. PR status testing was not mandated during the study period [16]; however, to define the TNBC population a confirmed negative PR status was required.
Defining surgical modality
Surgical records were linked to systemic treatment records and manually reviewed. As specific descriptions of surgical procedures were not available in the dataset, where patients had more than one record of “curative surgery”, the date closest to the next administration of chemotherapy was taken as date of definitive surgery for BC, as previous dates were considered to be a pre-operative tissue biopsy, rather than tissue biopsy performed prior to surgery.
Study objectives
The objectives of this study were to measure and compare TTS and TTC by region and ethnicity, to understand potential variation in treatment pathway timing by these demographic factors. TTC was calculated as the difference between date of surgery and the subsequent chemotherapy treatment date. Chemotherapy given within 180 days of surgery was considered part of the same treatment regimen, and patients with TTC longer than this period were excluded from the analysis. TTS was calculated as the time from the date of final neoadjuvant chemotherapy to surgery date. Patients with a surgery date within 180 days of neoadjuvant treatment were included under the assumption that surgery and the previous chemotherapy was part of the same treatment protocol.
NHS commissioning region was derived from organisation codes provided in the SACT dataset request. These commissioning regions were East of England, London, Midlands, North East & Yorkshire, North West, South East and South West. Patients were assigned to broad ethnicity groups according to UK census categories: White, Black, Asian, Mixed, Other (including “Unknown” ethnicity) [16].
TTC and TTS time periods were stratified into 0–30 days, 31–60 days, 61–90 days and > 90 categories. TTS/TTC periods were compared by region, ethnicity and Index of Multiple Deprivation, a measure of socioeconomic status, in the English TNBC population.
Statistical analysis
Statistical analysis was performed in R Studio v4.3.2. Chi-squared tests were used to test for significant differences in TTC/TTS by region and patient ethnicity. p-values < 0.05 were considered significant.
Results
1347 patients were included in the study after inclusion criteria were applied. Figure 1 shows numbers of patients excluded at each step.
Time to chemotherapy (TTC)
Of 17,666 total BC patients, 1176 (6.6%) TNBC patients with a record of adjuvant chemotherapy within 180 days of surgery were identified during the study period. Median TTC for the study cohort (n = 1176) was 45 days (IQR 29, 72): in the East of England median TTC was 47 days (IQR 24, 78, n = 180); London 43 days (IQR 20–58, n = 165); Midlands 50 days (IQR 36, 83, n = 221); North East and Yorkshire 45 days (IQR 27, 55, n = 249); North West 38 days (IQR 27, 55, n = 212); South East 49 days (IQR 30,79, n = 190); South West 49 days (34,79, n = 130). Median TTC for each commissioning region is summarised in Table 1. There were significant differences in TTC between commissioning regions (p < 0.001).
Time to surgery (TTS)
341 patients had a record of neoadjuvant chemotherapy prior to surgery. Median time from completion of neoadjuvant chemotherapy to surgery (TTS) was 18 days (IQR 8, 46) in the overall TNBC cohort. TTS was greatest in the East of England [28] and lowest in the Midlands [13]. TTS by region is shown in Table 1. Chi-squared tests did not show significant differences in the TTS period between regions (p = 0.3).
170 patients received both neoadjuvant and adjuvant chemotherapy and were assessed for both TTC/TTS.
TTC and TTS were split into four categories: 0–30 days, 31–60 days, 61–90 days and > 90 days and patients were grouped by commissioning region (Table 2). 13–22% of patients received adjuvant chemotherapy > 90 days after surgery, with the East of England, Midlands and the South West regions having the highest percentage (22%) of patients treated > 90 days after surgery, compared to the lowest percentage in the North West (13%). London and the North West had the highest percentages of patients receiving adjuvant chemotherapy within 30 days of surgery (33%), and the Midlands the lowest (20%). Median TTS varied from 13 days in the Midlands to 39 days in the East of England. Chi-squared test did not show significant regional differences in TTS period (p = 0.2).
TTC/TTS by ethnicity
TTC/TTS for each ethnic group is summarised in Table 3. Chi-squared tests did not suggest that ethnicity was a significant factor associated with differences in both TTC and TTS period (p > 0.05). TTC was longest in “Mixed Race” patients at 51 days and shortest in “Black” patients at 41 days respectively. “Mixed Race” and “Other” patients had a higher median TTC than the median of 45 days for the whole cohort. Chi-squared test did not suggest significant differences in either TTC or TTS period between patients of different ethnicity.
TTC and TTS time was categorised as 0–30, 31–60, 61–90 and > 90 days and patients were grouped by ethnic group (Table 4).”Black” patients were initiated adjuvant chemotherapy within 30 days of surgery at the highest rate (35%), whilst “Mixed Race” patients were least likely to be treated within this period (17%). “Other” patients were most likely to exceed the > 90-day period from surgery to adjuvant chemotherapy (25%), whilst “Black” made up 16% of patients taking > 90 days to receive adjuvant treatment.
Regarding TTS, “Asian” patients represented the highest proportion of patients receiving surgery within 30 days of completion of neoadjuvant chemotherapy (70%) and “Black” ethnicity treated at the lowest rate within 30 days (55%).
The difference in the proportion of “White”, “Black” and “Asian” patients who had chemotherapy within 90 days and after 90 days is summarised in Table 5. The proportion of patients treated < 90 days and > 90 days of surgery was comparable between each ethnicity group.
TTC by index of multiple deprivation
TTC was compared between patients relative to the Index of Multiple Deprivation (IMD), a measure of socioeconomic status. No significant differences in TTC by Index of Multiple Deprivation were observed in this analysis, with a non-significant difference of 11 days between median TTC. Results of analysis by IMD are given in Table 6.
Discussion
This retrospective cohort study included 1,347 TNBC patients treated with chemotherapy over a two-year period in England. Significant differences in time from surgery to adjuvant chemotherapy between NHS commissioning regions were observed in this cohort (p < 0.01). The analysis did not find ethnicity to be a significant factor affecting the TTC period (p > 0.05). Time from neoadjuvant chemotherapy to surgery, TTS, did not differ significantly between regions or ethnic groups in the analysis. This study was performed using national data during the 2014–2015, a period predating widespread genomic testing and when most BC patients were treated with adjuvant chemotherapy. We acknowledge that treatment pathways have changed significantly in BC since this time. Despite this limitation, the role of adjuvant chemotherapy is still pivotal to the treatment of BC, and therefore large population-level dataset analysis from this period can provide valuable insights into national treatment pathways that may not be achieved with other study approaches.
Previous studies have defined delay to adjuvant chemotherapy by different threshold values, such as 56 days or more following surgery [15]. This discrepancy poses questions about what the true definition of a delay in adjuvant treatment, which may be context dependent relative to the healthcare system in which patients are treated. In this study, we defined delayed TTC as greater than 90 days, as this clinically meaningful threshold has been identified by other researchers and used in other studies of this kind [10, 17, 18]. By this definition, patients in the East of England, Midlands and South West commissioning regions experienced the highest proportions of delayed TTC, with 22% of patients taking > 90 days to commence adjuvant chemotherapy. The Midlands had the highest median TTC in England, and the lowest proportion of patients initiating adjuvant chemotherapy with 30 days of surgery. The disparities observed between regions warrants further investigation to identify potential gaps in service provision leading to adjuvant chemotherapy delays within specific cancer treatment centres, and to fully understand where and for what reasons delays are occurring.
Ethnicity was not a significant factor influencing TTC in this study, however, due to the relatively low proportion of TNBC cancer patients relative to the whole BC population, ethnic minority groups formed small proportions of the cohort. Other studies have revealed differences in diagnostic pathways and stage at diagnosis in BC relative to ethnicity [19]; however, similar disparities were not observed in this analysis. This analysis did not support an association between Index of Multiple Deprivation and TTC period, suggesting patients were treated with adjuvant chemotherapy at comparable time irrespective of socioeconomic status.
Significant associations of TTC > 90 days with poorer survival outcomes have been reported by other authors [9,10,11]. Our study observed regional differences in TTC across the UK, but did not allow for robust survival analysis due to sample size limitations. As poorer outcomes would be expected in TNBC patients, the impact of a longer TTC period may be less pronounced than for patients with other hormone status. Gagliato et al. report TTC as inversely proportional to disease progression time [20] and suggest that early administration of adjuvant chemotherapy prolongs the disease-free period. These authors suggest that minimising the TTC period is important in achieving optimal outcomes, as this period is related to survival. A systematic review and meta-analysis by Yu et al. concluded that patients in high-risk groups, including TNBC patients, had poorer survival when initiating chemotherapy 61 < days after surgery compared to those who initiated adjuvant chemotherapy within 30 days of surgery [21]. These studies suggest that TTC is an important predictive factor affecting survival outcomes; however, other studies have found conflicting results.
An investigation of disease-free survival period in BC of all phenotypes has not found that administration of adjuvant chemotherapy had a significant impact on survival at 4 years, comparing those receiving adjuvant chemotherapy within 10 weeks or 10–18 weeks [22]. Another study found no significant survival benefit from initiating adjuvant chemotherapy within [23], with patients receiving chemotherapy within 21 days of surgery showing 82% 5-year overall survival compared to 84% 5 in those treated more than 21 days post-surgery. The Danish Breast Cancer Cooperative Group found treatment benefit from chemotherapy was equally effective when administered at any time within a 12-week period following surgery [24]. We acknowledge that these findings may differ in the TNBC population, and that the behaviour of BC varies significantly between patients due to a wide range of factors beyond treatments and given and treatment pathway timing.
An overall assessment of the evidence suggests that TTC may play an important role affecting survival outcomes in specific cohorts of BC patients. It is possible, due to the poor prognosis of TNBC patients, that TTC is a less significant factor than in patients with other hormone status. Despite the lack of statistical power to test for survival differences by TTC period in this analysis, the finding of disparity in TTC by region of England should be addressed to ensure equitable care for patients from all regions of England, considering evidence presented by Chavez et al., suggestive of poorer survival when TTC exceeds 90 days [21]. Evidence from other authors suggests that TTS period does not significantly impact survival, with reports of no difference in survival time relative to different TTS periods [25] [26]. It is also important to acknowledge that some publications have not identified positive associations between TTC and survival outcomes in TNBC patients [27, 28].
Limitations
Despite having a larger study cohort than previous studies investigating TTC/TTS in TNBC patients, small sample sizes in each regional and ethnic group may have limited study power in identifying statistically significant TTC/TTS differences between regions and patient ethnicity. Incomplete hormone status information likely reduced the identification of all TNBC patients, with TNBC accounting for 13% of all BC patients in the dataset, compared to the observed rate of ~ 15% in the wider BC population. This disparity is likely due to missing hormone status data, preventing identification of TNBC status for ~ 2% of patients.
The limited retrospective nature of this study did not allow for the analysis of current TTC differences of recent TNBC treatment data, therefore in order to obtain a better representation of current TTC, patterns should be compared with more recent records over a longer study period. Analysis into whether the effects of regional differences and ethnicity persist, or if they have changed, will be insightful for future treatment guidance, as BC treatment pathways today still employ adjuvant chemotherapy despite advances in targeted therapies since the 2014–2015 study period.
Other factors related to delays to adjuvant chemotherapy identified in previous studies include non-English language [29], postoperative complications [30] and inclusion in clinical trials [31]. With more detailed data, the influence of these factors could be investigated to understand the reasons for TTC delays in more detail. Analyses of non-surgical oncology service delays within the NHS would also be insightful into understanding the reasons for delayed treatments within the total BC and TNBC populations. Despite limitations, to the best of our knowledge this study is the first to analyse TTC and TTS for TNBC patients relative to ethnicity, geography and socioeconomic status in the English healthcare system.
Conclusions
This study found significant differences in median TTC by region of England, but did not find an association for ethnicity or socioeconomic status with TTC. TTS period did not differ significantly between any ethnic minority, socioeconomic or geographical patient groups in this analysis. Given other publications finding association between prolonged TTC and survival outcomes, clinicians should aim to reduce to initiate adjuvant chemotherapy in a timely manner, giving other evidence of poorer survival associated with TTC < 30 and < 60 days.
Data availability
No datasets were generated or analysed during the current study.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clinic 71(3):209–249
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72(1):7–33
The Global Cancer Observatory. united-kingdom-fact-sheets: World Health Organisation; 2021 [https://gco.iarc.fr/today/data/factsheets/populations/826-united-kingdom-fact-sheets.pdf.
Ismail-Khan R, Bui MM (2010) A review of triple-negative breast cancer. Cancer Control 17(3):173–176
De Giorgi U, Rosti G, Frassineti L, Kopf B, Giovannini N, Zumaglini F et al (2007) High-dose chemotherapy for triple negative breast cancer. Ann Oncol 18(1):202–203
Agarwal G, Nanda G, Lal P, Mishra A, Agarwal A, Agrawal V et al (2016) Outcomes of triple-negative breast cancers (TNBC) compared with non-TNBC: does the survival vary for all stages? World J Surg 40(6):1362–1372
Kumar P, Aggarwal R (2016) An overview of triple-negative breast cancer. Arch Gynecol Obstet 293(2):247–269
Ge J, Zuo W, Chen Y, Shao Z, Yu K (2021) The advance of adjuvant treatment for triple-negative breast cancer. Cancer Biol Med 19(2):187–201
Pomponio MK, Keele LJ, Fox KR, Clark AS, Matro JM, Shulman LN et al (2019) Does time to adjuvant chemotherapy (TTC) affect outcomes in patients with triple-negative breast cancer? Breast Cancer Res Treat 177(1):137–143
Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH (2016) Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol 2(3):322–329
Morante Z, Ruiz R, Araujo JM, Pinto JA, Cruz-Ku GDL, Urrunaga-Pastor D et al (2021) Impact of the delayed initiation of adjuvant chemotherapy in the outcome of triple negative breast cancer. Clin Breast Cancer 21(3):239–46.e4
Suleman K, Almalik O, Haque E, Mushtaq A, Badran A, Alsayed A et al (2020) Does the timing of surgery after neoadjuvant therapy in breast cancer patients affect the outcome? Oncology 98(3):168–173
Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K et al (2006) Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol 24(9):1357–1362
Seneviratne S, Campbell I, Scott N, Kuper-Hommel M, Round G, Lawrenson R (2014) Ethnic differences in timely adjuvant chemotherapy and radiation therapy for breast cancer in New Zealand: a cohort study. BMC Cancer 14:839
Heeg E, Marang-van de Mheen PJ, Van Maaren MC, Schreuder K, Tollenaar R, Siesling S et al (2020) Association between initiation of adjuvant chemotherapy beyond 30 days after surgery and overall survival among patients with triple-negative breast cancer. Int J Cancer 147(1):152–159
OfN S (2022) Ethnic group, England and Wales: Census 2021.
Smith-Graziani D, Lei X, Giordano SH, Zhao H, Karuturi M, Chavez-MacGregor M (2020) Delayed initiation of adjuvant chemotherapy in older women with breast cancer. Cancer Med 9(19):6961–6971
Cook P, Yin G, Ayeni FE, Eslick GD, Edirimanne S (2023) Does immediate breast reconstruction lead to a delay in adjuvant chemotherapy for breast cancer? A meta-analysis and systematic review. Clin Breast Cancer 23(5):e285–e295
Anna F, Becky W, Diana N, Jon S, Ruth HJ (2023) Relationship between ethnicity and stage at diagnosis in England: a national analysis of six cancer sites. BMJ Open 13(1):e062079
Altundag MK, Celik I, Ozisik Y (2000) Is there a range of time for initiation of adjuvant chemotherapy in patients with malignancy? Ann Oncol 11(9):1209
Gagliato Dde M, Gonzalez-Angulo AM, Lei X, Theriault RL, Giordano SH, Valero V et al (2014) Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 32(8):735–744
Buzdar AU, Smith TL, Powell KC, Blumenschein GR, Gehan EA (1982) Effect of timing of initiation of adjuvant chemotherapy on disease-free survival in breast cancer. Breast Cancer Res Treat 2(2):163–169
Shannon C, Ashley S, Smith IE (2003) Does timing of adjuvant chemotherapy for early breast cancer influence survival? J Clin Oncol 21(20):3792–3797
Cold S, During M, Ewertz M, Knoop A, Moller S (2005) Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish breast cancer cooperative group (DBCG). Br J Cancer 93(6):627–632
Al-Masri M, Aljalabneh B, Al-Najjar H, Al-Shamaileh T (2021) Effect of time to breast cancer surgery after neoadjuvant chemotherapy on survival outcomes. Breast Cancer Res Treat 186(1):7–13
Sanford RA, Lei X, Barcenas CH, Mittendorf EA, Caudle AS, Valero V et al (2016) Impact of time from completion of neoadjuvant chemotherapy to surgery on survival outcomes in breast cancer patients. Ann Surg Oncol 23(5):1515–1521
Eastman A, Tammaro Y, Moldrem A, Andrews V, Huth J, Euhus D et al (2013) Outcomes of delays in time to treatment in triple negative breast cancer. Ann Surg Oncol 20(6):1880–1885
Larson KE, Grobmyer SR, Karafa M, Pratt D (2018) Time to treatment and survival in triple negative breast cancer patients receiving trimodality treatment in the United States. Cancer Treat Res Commun 16:32–37
Tang A, Mittal A, Mooney CM, Khoury AL, Chiang A, Lai N et al (2022) Factors delaying chemotherapy in patients with breast cancer at a safety-net hospital. J Natl Med Assoc 113(6):706–712
Kim IY, Kim BR, Kim YW (2015) Factors affecting use and delay (>/=8 weeks) of adjuvant chemotherapy after colorectal cancer surgery and the impact of chemotherapy-use and delay on oncologic outcomes. PLoS ONE 10(9):e0138720
Meyer C, Bailleux C, Chamorey E, Schiappa R, Delpech Y, Dejode M et al (2022) Factors involved in delaying initiation of adjuvant chemotherapy after breast cancer surgery. Clin Breast Cancer 22(2):121–126
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Contributions
LM: responsible for study design, analysis, interpretation of results, production of all figures and writing of first draft manuscript. LS: assistance with study design, responsible for data analysis, redrafting and editing of final manuscript, submission of paper to journal. RR: provided review of manuscript with suggested changes and clinical guidance CH: provided clinical feedback on manuscript CC: provided clinical feedback on manuscript EM: provided clinical feedback on manuscript, IP: provided clinical feedback on manuscript SF: provided clinical feedback on manuscript PC: project inception and management, clinical guidance, providing comments on draft manuscript. All authors reviewed the manuscript and provided feedback prior to submission.
Corresponding author
Ethics declarations
NM reports honoraria from Gilead, Novartis and Roche. SF reports honoraria from Astra Zeneca, Chugai, Gilead, Lilly, Novartis, Pfizer, Roche & Seagen. EM reports honoraria from Lilly and Celltrion. IP reports honoraria from Novartis and educational sponsorship from Roche, Novartis and Pfizer. CC reports sponsorship from Gilead, B. Braun and Novartis. PC reports grant funding from the National Institute for Health and Care Research LS, LM, RR, EM and CH report no disclosures/conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mirza, L., Steventon, L., Roylance, R. et al. Regional differences in neo/adjuvant chemotherapy timing in patients with early-stage triple-negative breast cancer in England. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07480-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10549-024-07480-x