Abstract
We determined the frequency and mutational spectrum of BRCA1 and BRCA2 in a series of patients at high risk for developing breast cancer from Brazil. A total of 1267 patients were referred for BRCA genetic testing, and no obligation of fulfilling criteria of mutation probability methods for molecular screening was applied. Germline deleterious mutations in BRCA1/2 (i.e., pathogenic/likely pathogenic variants) were identified in 156 out of 1267 patients (12%). We confirm recurrent mutations in BRCA1/2, but we also report three novel mutations in BRCA2, not previously reported in any public databases or other studies. Variants of unknown significance (VUS) represent only 2% in this dataset and most of them were detected in BRCA2. The overall mutation prevalence in BRCA1/2 was higher in patients diagnosed with cancer at age > 35 years old, and with family history of cancer. The present data expand our knowledge of BRCA1/2 germline mutational spectrum, and it is a valuable clinical resource for genetic counseling and cancer management programs in the country.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
BRCA1 and BRCA2 are two major breast cancer susceptibility genes in the context of large, multiple‐case families, segregating both early onset breast cancer and ovarian cancer [1,2,3,4]. Pathogenic variants in these genes account for 20%–40% of the familial breast cancer cases [5]. Female carriers of BRCA1/2 mutations have an increased lifetime risk of developing both breast and ovarian cancers [6,7,8]. Currently, germline genetic screening for BRCA1/2 mutations has been routinely applied for high-risk patients, being an essential tool for cancer prediction and clinical management. The BRCA1/2 mutation carriers can benefit from intensive surveillance, prophylactic surgery, and chemoprevention, reducing their risk of developing breast cancer [9,10,11]. Additionally, clinical trials of targeted drugs, such as poly ADP-ribose polymerase inhibitors, shed light on the treatment promise for advanced breast cancer patients who carry BRCA1/2 mutations [12, 13]. Integration of genetic counseling and testing is then paramount for diagnosis of hereditary breast and ovarian cancer syndrome in mainstream oncology.
Since the identification of BRCA gene, extensive efforts have been dedicated to its sequence data analysis. However, the clinical interpretation of variants in BRCA1/2 sometimes can be challenging. To date, more than 20,000 unique variants, including missense, nonsense, frameshift, and splicing variants as well as large rearrangements have been described in both genes (www.brcaexchange.org). While some of them can be confidently predicted to be pathogenic since they affect the structure and function of the gene, a significant proportion of them are rare missense with unknown functional consequences. Moreover, the prevalence and spectrum of BRCA1/2 mutations is extremely variable among certain populations and ethnic groups [14,15,16]. Several studies have been conducted to evaluate the epidemiologic characteristics of BRCA1/2 mutations in diverse populations, however, most of them focused on white populations from Europe and North America, Asian, and African American populations. In particular, the Brazilian population has one of the most heterogeneous genetic constitutions in the world with a predominant tri-hybrid composition (Native Americans, Europeans, and Africans) in an extensive admixture [17]. Although the number of studies increased in the past few years [18, 19], the mutational spectrum of BRCA1/2 in the Brazilian population remains largely unknown. Usually, the sample size of the studies is typically small, and little is known about the prevalence of in BRCA1/2 germline mutations in Brazilian patients. Hence, there is a need for better understanding the germline mutational landscape of these high-penetrance genes and cancer risk prediction, so that appropriate genetic counseling and clinical management programs could be implemented. In this study, we present a comprehensive analysis of BRCA1/2 germline mutations from a cohort of 1,267 patients at high risk for developing breast cancer examined in a diagnostic routine.
Patients and methods
Casuistic
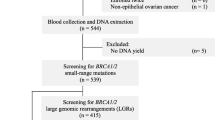
This is a retrospective, observational study that compiled clinical and molecular data of BRCA genetic testing results from patients investigated routinely in a private laboratory from Brazil (Diagnostics of America S.A.—DASA), between January 2017 and March 2019. A total of 1267 consecutive, unrelated individuals were referred for molecular screening either because of personal or family history of breast and/or ovarian cancer. The genetic testing was performed upon a medical request, which is linked to the patient risk stratification for hereditary breast and ovarian cancer. The clinicians followed international guidelines of a selected panel of experts who define criteria for testing individuals at high risk for hereditary breast and ovarian cancer [20, 21]. Briefly, the genetic testing was offered to patients who met at least one of the following criteria:
-
(i)
Individual from a family with a known deleterious BRCA1 and BRCA2 mutation;
-
(ii)
Personal history of breast cancer and \(\ge 1\) of these: (a) diagnosed at age \(\le\) 45 years; (b) diagnosed at age ≥ 50 years with ≥ 1 close blood relatives with breast cancer at age 50 years and/or ≥ 1 close blood relatives with epithelial ovarian cancer at any age; (c) two breast primaries when first breast cancer diagnosis occurred at age ≤ 50 years; (d) diagnosed at age ≤ 60 years with a triple negative breast cancer; (e) diagnosed at age ≤ 50 years with a limited family history; (f) diagnosed at any age, with ≥ 2 close blood relatives with breast and/or epithelial ovarian cancer at any age; (g) diagnosed at any age with ≥ 2 close blood relatives with pancreatic cancer at any age; (h) close male blood relative with breast cancer; (i) individual of ethnicity associated with higher mutation frequency (e.g., Ashkenazi Jewish), (j) personal history of epithelial ovarian cancer, male breast cancer, or pancreatic cancer at any age with ≥ 2 close blood relatives with breast and/or ovarian cancer and/or pancreatic cancer at any age;
-
(iii)
No personal history of breast cancer, but with a family history including \(\ge 1\) of these: (a) first- or second-degree blood relative meeting any of the above criteria; (b) third-degree blood relative with breast cancer and/or ovarian cancer with ≥ 2 close blood relatives with breast cancer (≥ 1 with breast cancer at age ≤ 50 years) and/or ovarian cancer.
Clinical information was collected from the genetic test requisition form, which was filled by the patients, and included: sex, age at cancer diagnosis or referral for genetic testing, history of unilateral or bilateral breast cancer, triple negative breast cancer status, personal history of other types of cancer, and family history of cancer.
This study was approved by the Ethics Committee from Hospital 9 de Julho (CAAE: 53,253,821.8.0000.5455), and all patients provided an informed consent for genetic testing.
BRCA genetic testing and variants analysis
Genomic DNA samples were extracted from peripheral blood cells following standard procedures. All patients were subjected to a comprehensive BRCA testing (full BRCA sequencing and multiplex ligation-dependent probe amplification—MLPA). The genetic testing was performed using different methodologies, including full gene analysis by Sanger or Next Generation Sequencing (NGS), and MLPA for analysis of large genomic rearrangements (LGR). In particular, the NGS-based capture method used was either the Ion AmpliSeq BRCA1/2 Panel (ThermoFischer, USA) or the Hereditary Cancer Panel designed by SOPHiA GENETICS (Switzerland), that encompasses the entire coding sequences, and each intron/exon boundaries of BRCA1/2 genes. Sequencing data were analyzed using the SOPHiA DDM™ software with a specific algorithm for variant calling and annotation, which also include LGR detection. LGR were confirmed by MLPA (SALSA MLPA P002-D1 and P045-B3—MRC Holland); the amplified products were electrophoretically separated using the ABIPrism310 genetic analyzer and interpreted with the Coffalyser analysis software (MRC Holland).
The Human Genome Variation Society (HGVS) nomenclature guidelines (http://varnomen.hgvs.org/) was used for variants annotation, and the ClinVar database (www.ncbi.nlm.nih.gov/clinvar/) was used to determine the biological significance of all reported variants. The detected variants were classified according to the American College of Medical Genetics and Genomics (ACMG) guidelines [22] as Pathogenic (P), Likely Pathogenic (LP), Benign (B), Likely Benign (LB), or Variants of Unknown Significance (VUS). The common variants (B/LB), i.e., those frequently reported in curated databases were disregarded from this study. Sanger sequencing was performed to validate all clinically relevant variants (P, LP, and VUS). For novel variants, Breast Cancer Information Core (http://research.nhgri.nih.gov/bic), BRCA Share (formerly known as UMD, http://www.umd.be/), LOVD (http://www.lovd.nl/3.0/home), ARUP (http://arup.utah.edu/database/ BRCA/) and BRCA Exchange (http://brcaexchange.org/) databases were also checked. As an additional data source for variants classification, we also consulted the Mastermind database (https://mastermind.genomenon.com/), and reports in the literature from the Brazilian population and worldwide studies [19, 23]. Importantly, for supporting evidence of pathogenicity of novel variants and VUS, we used the Alamut Visual Plus™ software (SOPHiA GENETICS, Switzerland) that assesses both the probability of protein sequence damage, and de novo creation of splice sites, based on NNSplice and MaxEnt algorithms. Further, to estimate the impact of novel variants and VUS on protein structure we also specify three evidence categories (population frequency data, variant type and location, and case-level data) as recommended by Harrison 2019 [24].
Results
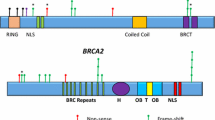
Out of the 1267 patients referred for molecular screening, 1080 had a negative result, and the remaining 187 individuals were either positive for a clinically relevant variant (P/LP) or VUS in the BRCA gene; the corresponding frequency of P/LP variants and VUS in our cohort were 12% and 2%, respectively (Supplementary Fig. 1A). The Supplementary Table 1 shows that, as expected, the largest proportion of patients investigated were in fact females (98%). Among the individuals with P/LP variants, 93 (60%) of them carried a mutation on BRCA1, and 63 (40%) carried a mutation on BRCA2. Regarding the frequency of VUS, no significant difference was observed between both genes (Supplementary Fig. 1B). The distribution of the different types of mutations on BRCA1/2 is shown in Supplementary Fig. 1C; nonetheless, frameshift mutations are the most frequent deleterious variant in both genes, accounting for nearly half of all cases. Of note, three distinct and novel presumably disease-causing variants were detected in BRCA2 (Supplementary Fig. 1D). The spectrum of all BRCA1/2 mutations identified in our cohort is presented in Tables 1 and 2.
The most frequent P/LP variants on BRCA1/2, here defined as a mutation found in three or more individuals, are unequally distributed in the five different regions of Brazil (Fig. 1). The BRCA1 c.5266dup (p. Gln1756ProfsTer74) variant, highlighted in bold, was the most common in our cohort, detected in 20 individuals and in all geographical areas of the country. In particular, the novel variants, each of them representing a single case, were observed in the Midwest, Southeastern, and South region of Brazil. However, the number of patients referred for BRCA genetic testing is not equal in the country (Supplementary Table 2). The North region has the lowest number of individuals investigated in this dataset, even though the proportion of negative cases, P/LP variants and VUS was very similar among the five regions (Supplementary Table 3).
Geographical distribution of the most frequent BRCA1/2 mutations in Brazil. A total of eleven mutations are displayed in the map, and these were defined as most frequent because they were found in three or more individuals in the present cohort. The BRCA1 c.5266dup (p. Gln1756ProfsTer74) variant, highlighted in bold, was the most common, detected in 20 individuals and in all geographical areas of the country
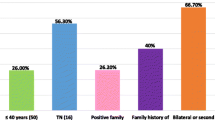
Overall, the mean age of all patients investigated was 42 years old (Fig. 2A). The mutation prevalence in patients with cancer, by age group, was particularly high on women aged between 36 and 40 years old (Fig. 2B); late-onset cancer (\(\ge\) 45 years old) indeed represents most cases, as shown on Fig. 2C. Also, the mutation prevalence was significantly higher in patients who also had a family history of cancer (Fig. 2D). Supplementary Table 4 presents a more detailed description of mutation prevalence in patients with or without diagnosis of breast and/or ovarian cancer, according to family history and the corresponding frequency of P/LP, VUS and negative results in our cohort. Unilateral breast cancer represents the largest number of cases in our cohort (67% and 81% in patients with P/LP variants and VUS, respectively). Besides, patients with P/LP variants have approximately 3 times more bilateral breast cancer (18%) when compared to those with VUS (6%) (Supplementary Tables 5, 6 and 7). Notably, patients with P/LP variants who have a first-degree family history with a diagnosis of ovarian cancer add up to more than double the number of patients with VUS.
Characteristics of BRCA1/2 mutation carriers in this study. A The mean age at cancer diagnosis of all patients investigated was 42 years old. B Mutation prevalence in patients with cancer by age group. C Frequency of patients with early (\(\le\) 35 years old) and late-onset cancer (\(\ge\) 45 years old). D Frequency of patients with personal history of cancer at diagnosis and with family history of cancer
Discussion
In the present study, we evaluated the frequency and mutational spectrum of BRCA1/2 in a series of patients being at high risk for hereditary breast and ovarian cancer. No obligation of fulfilling criteria of mutation probability methods for molecular screening was applied. In our cohort, 12% (156/1267) of the patients carried a deleterious germline BRCA mutation (93 BRCA1 and 63 BRCA2), being the vast majority very rare, found in only one or two individuals. Particularly, the most frequent BRCA1 mutation, accounting for 21.5% of all BRCA1 mutations, was the c.5266dup (p.Gln1756ProfsTer74), while the BRCA2 c.2808_2811del (p.Ala938ProfsTer21), accounting for 12.7%, was the most frequent of all BRCA2 mutations.
The BRCA1 c.5266dup (p.Gln1756ProfsTer74) was found in 20 individuals and among all geographical regions in Brazil. This mutation, also known as 5382insC or 5385insC, is a known founder mutation in individuals of Ashkenazi Jewish ancestry [25], and present at appreciable frequency in several European countries [26]. Noteworthy, 19 individuals have European descent between those with the BRCA1 c.5266dup mutation. The median age at breast cancer diagnosis in this series was 35 years old, and four individuals were discriminated as mutation carriers without breast, ovarian or any other type of cancer. Three out four carriers had a family history of breast or ovarian cancer in at least one first- and second-degree relatives at young age. Unilateral triple negative breast cancer was seen in 6/20 and three patients presented bilateral breast cancer.
The BRCA2 c.2808_2811del (p.Ala938ProfsTer21) was found in 8 individuals from the Southern and Southeastern regions of Brazil. Five women with unilateral breast cancer harboring this specific mutation were detected in this series. The median age at breast cancer diagnosis in these cases was 35 years old, which include one male carrier at the age of 40, with family history of hereditary breast and ovarian cancer syndrome, and two females’ carriers at the age of 23 and 61. The 23 years old female has a family history of breast cancer that included three cases of first- and second-degree relatives. An additional high burden of other types of cancers has been observed in the families with BRCA2 c.2808_2811del carriers, in particular, an enrichment of prostate cancer. It is relevant to mention that among all BRCA2 mutations a few were seen in several populations in different ethnic groups, but the c.2808_2811del is in the top 10 most frequent mutations detected between all ethnicities [27]. Recently, the BRCA2 c.2808_2811del was described as the second most common BRCA2 variant in a Brazilian populational study [19].
Notably, considering the most frequent P/LP variants in this dataset (Fig. 1), the three most common are in accordance with a recent worldwide BRCA1/2 mutational spectrum report, which listed BRCA1 c.5266dup, BRCA1 c.3331_3334del and BRCA2 c.2808_2811del, among the top 5 BRCA1/2 mutations in Brazil [27]. With particular interest, we highlight a BRCA2 mutation in our cohort that are strongly associated with the African American population. The c.6405_6409del (p.Asn2135LysfsTer3) was the second most common BRCA2 mutation, and it was present in six individuals from the Southern and Southeastern regions of Brazil. Three women with unilateral breast cancer, two triple negative and a 60 years old patient with ovarian cancer harbor this pathogenic variant. Within this group there was also a female carrier at the age of 22 with family history of hereditary breast cancer. Of note, the c.6405_6409del was found among the 10 most common mutations in Brazil, African American and South/Central America [27]. It is worth mentioning that the geographical distribution of BRCA1/2 mutations in Brazil, as shown in Fig. 1 and Supplementary Tables 2 and 3, highlights the unequal frequency of patients referred to genetic testing and access of supplementary health in the country. The Brazilian Society of Medical Genetics (https://www.sbgm.org.br/) points out that one third of the country`s geneticists are located in the state of São Paulo, which certainly influences molecular investigation and treatment of the patients.
Nearly all P/LP in BRCA1/2 described here were previously reported, except for three novel variants in BRCA2: (i) a frame shift mutation starting at codon Asn1201 [c.3601_3602delinsT; p.(Asn1201SerfsTer8)] detected in a 64 years old woman with breast cancer, and family history for breast and prostate cancer; (ii) a 1 bp duplication in exon 11 that interrupts the reading frame prematurely at position 1220[c.3659dup; p. (Tyr1220Ter)], detected in a 60 years old woman, African descent with bilateral triple negative breast cancer, and no family history for breast, ovarian or other cancer type; and (iii) a 1 bp deletion in exon 21 leading to a frameshift [c.8682del; p.(Val2895PhefsTer14)] effect in a 28 years old Italian descendant woman with bilateral triple negative breast cancer and familial history of Hereditary breast, ovarian and colon cancer.
Regarding the frequency of VUS, this class of variant represent only 2% (31/1267) in our cohort. VUS impose a challenge relating to the management and surveillance of carriers as well as in risk assessment. To categorize these variants, six different in silico tools were applied (i.e., phyloP; Grantham dist; Align GVGD; SIFT and Mutation Taster), and their classification was also based on consulted databases and complementary sources as previously described. A total of 31 unique VUS were identified tin the patients (14 and 17 in BRCA1 and BRCA2, respectively), and none of them was detected neither at carriers of BRCA1/2 P/LP variants nor present more than once. Out of the 14 BRCA1 VUS, 13 are reported on ClinVar database to have unknown clinical significance, and only one variant has no records on public databases (BRCA1 c.3975G > T) (Table 1). Interestingly, we identified one non-coding VUS in BRCA1 localized in the 3´UTR region, the c.*291C > T, which was detected in a 57 years old woman with unilateral breast cancer and family history of breast cancer. A functional luciferase assay previously showed that the c.*291C > T increased BRCA1 3'UTR activity [28]. Although there is an increasing number of data associating germline non-coding variants with higher cancer risk, a co-segregation analysis was not possible to be made because the VUS was reported only in this woman. It is noteworthy that this non-coding variant was only described in breast cancer cases [29]. Among the 17 BRCA2 VUS, 14 are reported on ClinVar database to have unknown clinical significance, while 3 of them have no records on public databases (BRCA2 c.67 + 25 T > C, c.3045G > T and c.8755G > T) (Table 2). In particular, the BRCA2 c.8755G > T variant was previously described in a Brazilian series where it also was provisionally classified as VUS [18]; in silico tools predicts that this variant exerts a possible effect nearest the splice site. Considering that there is a degree of inconsistency between these in silico predictions tools, it is impossible to draw any conclusions on the pathogenicity of VUS. Nonetheless, an integrated strategy which will include co-segregation analysis, tumor pathology data, as well as functional assays is needed to complete a comprehensive assessment of pathogenicity of these variants.
The characteristics of BRCA1/2 mutation carriers in our cohort (i.e., the overall mutation prevalence was higher in patients diagnosed with cancer > 35 years old, and with a family history of cancer) were similar to other studies. Although it was expected that patients with a BRCA1/2 mutation were more likely to have bilateral breast cancer, in the current study unilateral breast cancer represent the largest number of cases. Nonetheless, patients with P/LP variants in BRCA1/2 have approximately 3 times more bilateral breast cancer when compared to those with VUS. Different definitions of familial breast cancer and genetic testing methods for BRCA1/2 mutations between the studies may influence the results. Currently, the National Comprehensive Cancer Network (NCCN) guidelines are the most widely used criteria for testing and inform insurance coverage decisions. Even though the criteria have expanded over time to be more inclusive, recent data suggest that expansion of testing may be appropriate [30, 31]. The mutation detection rate in patients with breast cancer tested based on NCCN guidelines varied widely depending on the type and number of criteria met. In general, BRCA detection rates were significantly increased when the reason for testing was age ≤ 45 at time of diagnosis or having a known family history of a BRCA mutation, but specific clinical scenarios such as triple negative breast cancer status also have been associated with a high risk for BRCA mutations. We followed the NCCN guidelines for genetic testing referral in our patient cohort, which may explain the higher diagnostic yield (12%) when compared to the estimated frequency of 5–10% of all hereditary breast and ovarian cases attributed to P/LP germline mutations in BRCA1 and BRCA2 [32].
In summary, the present data expand our knowledge of the frequency and BRCA1/2 germline mutational spectrum in Brazil, being a valuable clinical resource for genetic counseling and cancer management programs in the country. Also, based on the increase interest for BRCA genetic testing among individuals who are at high risk of carrying a mutation, our data may help provide guidelines in the future for patients with breast cancer who should undergo molecular screening.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Narod SA, Foulkes WD (2004) BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer 4:665–676
Lakhani SR et al (2005) Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res 11:5175–5180
Turner N, Tutt A, Ashworth A (2004) Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer 4:814–819
Venkitaraman AR (2002) Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108:171–182
Lee A, Moon BI, Kim TH (2020) BRCA1/BRCA2 pathogenic variant breast cancer: treatment and prevention strategies. Ann Lab Med 40:114–121
King MC, Marks JH, Mandell JB (2003) Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 1979(302):643–646
Kuchenbaecker KB et al (2017) Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA—J Am Med Assoc 317:2402–2416
Boddicker NJ et al (2021) Risk of late-onset breast cancer in genetically predisposed women. J Clin Oncol 39:3430–3440
Anderson K et al (2006) Annals of internal medicine article or a BRCA2 mutation. Ann Intern Med 144:397–406
Salhab M, Bismohun S, Mokbel K (2010) Risk-reducing strategies for women carrying brca1/2 mutations with a focus on prophylactic surgery. BMC Womens Health 10:1–10
Liu YL et al (2022) Risk-reducing bilateral Salpingo-Oophorectomy for ovarian cancer: a review and clinical guide for hereditary predisposition genes. JCO Oncol Pract 18:201–209
Liu X et al (2021) Efficacy and safety of PARP inhibitors in advanced or metastatic triple-negative breast cancer: a systematic review and meta-analysis. Front Oncol 11:1–9
Yang Y et al (2020) The efficacy and safety of the addition of poly ADP-ribose polymerase (PARP) inhibitors to therapy for ovarian cancer: a systematic review and meta-analysis. World J Surg Oncol 18:517–523
Hall MJ et al (2009) BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer 115:2222–2233
Janavičius R (2010) Founder BRCA1/2 mutations in the Europe: Implications for hereditary breast-ovarian cancer prevention and control. EPMA Journal 1:397–412
Fackenthal JD, Olopade OI (2007) Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat Rev Cancer 7:937–948
Pena SDJ, Santos FR, Tarazona-Santos E (2020) Genetic admixture in Brazil. Am J Med Genet C Semin Med Genet. https://doi.org/10.1002/ajmg.c.31853
Fernandes GC et al (2016) Prevalence of BRCA1/BRCA2 mutations in a Brazilian population sample at-risk for hereditary breast cancer and characterization of its genetic ancestry. Oncotarget 7:80465–80481
Palmero EI et al (2018) The germline mutational landscape of BRCA1 and BRCA2 in Brazil. Sci Rep 8:1–10
Achatz MI et al (2020) Recommendations for advancing the diagnosis and management of hereditary breast and ovarian cancer in Brazil. J Glob Oncol 6:439–452
Daly MB et al (2021) Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 2.2021. JNCCN J Nat Compr Cancer Netw 19:77–102
Richards S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med 17:405–424
Li X et al (2018) Mutational spectrum in a worldwide study of 29,700 families with BRCA1 and BRCA2 mutation. Hum Mutat 39:593–620
Harrison SM et al (2020) 2020 Overview of specifications to the ACMG/AMP variant interpretation guidelines. Curr Protoc Hum Genet 103:1–20
Nanda R et al (2005) Genetic testing in an ethnically diverse cohort of high-risk women. JAMA 294:1925
Hamel N et al (2011) On the origin and diffusion of BRCA1 c.5266dupC (5382insC) in European populations. Eur J Hum Genet 19:300–306
Rebbeck TR et al (2018) Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat 39:593–620
dos Santos ES et al (2018) Non-coding variants in BRCA1 and BRCA2 genes: potential impact on breast and ovarian cancer predisposition. Cancers (Basel) 10:1–21
Brewster BL et al (2012) Identification of fifteen novel germline variants in the BRCA1 3′UTR reveals a variant in a breast cancer case that introduces a functional miR-103 target site. Hum Mutat 33:1665–1675
Yang S et al (2018) Underdiagnosis of hereditary breast and ovarian cancer in medicare patients: genetic testing criteria miss the mark. Ann Surg Oncol 25:2925–2931
Beitsch PD et al (2018) Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol 37:453–460
Yoshida R (2021) Hereditary breast and ovarian cancer (HBOC): review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer 28:1167–1180. https://doi.org/10.1007/s12282-020-01148-2
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
PM, FM, EP, GG: conceptualization. BS, VN: methodology. MA, SM, PMM, JSS: validation. PM: formal analysis. PM: data curation. DV, EP, EB, TLC: writing—review & editing. IZ: supervision. MPM: Project administration. S-NC: funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the ethics committee from Hospital 9 de Julho (CAAE: 53253821.8.0000.5455), and all patients provided an informed consent for genetic testing.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mazzonetto, P., Milanezi, F., D’Andrea, M. et al. BRCA1 and BRCA2 germline mutation analysis from a cohort of 1267 patients at high risk for breast cancer in Brazil. Breast Cancer Res Treat 199, 127–136 (2023). https://doi.org/10.1007/s10549-023-06892-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06892-5