Abstract
Background
The tumor immune microenvironment plays a critical role in the prognosis and outcome of breast cancers. This study examined the role of tumor-infiltrating lymphocytes (TILs), CD8+, FOXP3+ lymphocytes, PD-L1 expression, and other clinicopathological parameters in HER2+ breast cancer and correlate with tumor response to neoadjuvant therapy.
Methods
We included 173 HER2+ patients treated with neoadjuvant HER2-targeted chemotherapy regimens from 2010 to 2016. 67 cases had biopsy blocks to evaluate TIL, CD8, FOXP3, and PD-L1 immunohistochemistry staining. Tumors were classified as pCR vs non-pCR group. Clinicopathological parameters, TIL, CD8+ and FOXP3+ cell count, and PD-L1 expression were correlated with pCR rate.
Results
Univariate analyses showed that pCR rate was significantly correlated with low PR, low ER, high Ki-67, high FOXP3, HER2 IHC3+ , high HER2 ratio and copy number. By multivariate analysis, Ki-67 was the only variable significantly correlated with pCR. PD-L1 expression was detected in 9.2% cases. TIL hotspot has a non-significant correlation with pCR rate (p = 0.096).
Conclusions
High Ki-67 is a strong predictor for pCR in HER2+ breast cancer. TIL and FOXP3 T cells may play a role in tumor response in HER2+ cancer. PD-L1 is expressed in a subset of HER2+ breast cancer, supporting a role of immunotherapy in treating a subset of HER2+ breast cancers. The role of PD-L1, TIL, and other markers of immunogenicity as predictors of response to neoadjuvant chemotherapy in HER2+ breast cancer should be further evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer continues to be the most commonly diagnosed cancer and second leading cause of cancer-related death among women in the United States [1]. Breast cancer is a heterogeneous group of disease and different subtype of breast cancer has different tumor biology and prognosis [2,3,4,5,6,7,8]. Approximately 20–30% of breast cancers overexpress the human epidermal growth factor receptor 2 (HER2) [9]. HER2-overexpressing breast cancers have a high likelihood of achieving pathologic complete response (pCR) to neoadjuvant HER2-directed regimens. The pCR rates in large neoadjuvant trials incorporating chemotherapy and anti-HER2 therapy ranged from 46 to 66% [10, 11]. The response to neoadjuvant chemotherapy is prognostic for patients with HER2+ breast cancer. For patients who do not achieve pCR, we can employ novel strategies to help minimize the risk of recurrence [12]. The KATHERINE trial showed that patients who did not achieve a pCR to neoadjuvant HER2-directed therapy had improved 3-year disease-free survival by 11% by switching to trastuzumab emtansine in the adjuvant setting as opposed to continuing with trastuzumab alone [13].
Given the increasingly important value of pCR in HER2+ breast cancer as both a prognostic marker and a tool to aid in decision-making about adjuvant therapies, understanding the role that immune cells and tumor-infiltrating lymphocytes (TILs) play in predicting pCR is of great interest and clinical relevance. There are several recent clinical trials that have shed light on this issue. In the HER2+ cancer cohort of the GeparSixto trial, among patients classified as having lymphocyte predominant breast cancer, pCR rates were higher than those who had lower levels of lymphocytic infiltration (64% vs 27%), and the addition of carboplatin to the treatment increased the pCR rate in the lymphocyte predominant patients but reduced it in non-lymphocyte predominant patients [14]. In the NeoALTTO study, patients with > 5% TILs were more likely to achieve pCR than those with lower levels of TILs, and there was a linear association between TIL percentage and event-free survival [15]. However, the results were not consistent among the studies. Some studies showed no association between response to therapy and TIL in multivariate analysis [16, 17]. To better understand the role of the immune system of HER2-overexpressing breast cancer, we examined the role of TILs, CD8+ and FOXP3+ lymphocytes, and PD-L1 expression in the tumor response to neoadjuvant therapy in HER2+ breast cancer.
Patient information and methods
Patient information
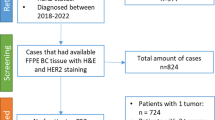
This study was approved by the Emory University Institutional Review Board. A total of consecutive 173 HER2+ breast cancer cases treated with neoadjuvant therapy from 2010 to 2016 were identified from our institution. All cases were either HER2 IHC 3 + or HER2 gene amplified by FISH analysis according to the 2018 ASCO/CAP recommendations [18]. Among the 173 cases, 111 had H&E slides for morphology evaluation and 67 had tissue blocks available for immunohistochemistry (IHC) evaluations. Clinicopathologic parameters, including age at diagnoses (age), tumor size before treatment, estrogen receptor (ER) and progesterone receptor (PR) H score (% × intensity), Ki-67, HER2 IHC, HER2/CEP17 ratio (HER2 ratio) and HER2 copy number (HER2 copy #), were collected from pathology reports and patient charts. Nuclear grade (1/2 vs 3), Nottingham grade (I/II vs III), mitosis/10 high-power field (HPF) and TILs were evaluated. TILs were evaluated as average TIL and hot spot TIL. Average TIL was defined as the average TIL percentage in the entire intratumoral stroma. Hot spot TIL was defined by the area of high-density TIL (more than 50%) divided by the entire intratumoral stroma (Fig. 1).
All patients received neoadjuvant therapy composed of HER2-targeted therapy (trastuzumab or pertuzumab or both) and chemotherapy. Biopsies from these patients were evaluated, and features were correlated with tumor response to neoadjuvant therapy. Tumor response was evaluated on excisional specimens and classified as pCR vs non-pCR; pCR was defined as no invasive carcinoma and no tumor thrombi in lymphovascular channels in the breast and no metastasis in the axillary lymph nodes at the time of surgery.
Immunohistochemistry
All biopsies were fixed in 10% neutral buffered formalin within an hour and for 6–72 hours. Formalin-fixed paraffin-embedded (FFPE) blocks were cut to 4-µm thickness and placed on a positively charged glass slide. Unstained slides went through antigen retrieval in citrate buffer for 20 min (min) at pH 6.0 and were then stained for ER (1:50; Dako, Carpenteria, California), PR (1:400; Dako), HER2 (HercepTest Her2 antibody; Dako), CD8 (clone C8/144B, 1:100; Dako), or PD-L1 (clone E1L3N, 1:200; Cell Signaling, Beverly, CA) using an automated Leica Bond III stainer (Leica Biosystems, Buffalo Grove, IL). FOXP3 (clone 236A/E7, 1:100; Abcam) staining was performed using a Dako link48 autostainer (Dako Biosystems, Santa Clara, CA). ER and PR nuclear staining and HER2 membrane staining were evaluated. CD8+ and FOXP3+ cells were counted within the tumor and the surrounding area (within 1 mm from tumor-invading front). The average absolute count of positive staining cells in 10 HPF was used to correlate with pCR rate (Fig. 2). PD-L1 expression levels in tumor cells and stroma were evaluated by Allred score (intensity × percentage, Fig. 3). PD-L1 positivity was defined as any PD-L1 staining in tumor cells or stroma. Appropriate positive controls were used in all cases.
Statistical analysis
Clinicopathologic characteristics were summarized by count and percentage for categorical variables, and by mean and standard deviation for continuous variables. Continuous variables included age, tumor initial size, mitosis, ER and PR H scores, Ki-67, HER2 copy #, ratio, CD8, FOXP3, stromal PD-L1, tumor PD-L1, average TIL, and hot spot TIL. Categorical variables included nucleus grade, Nottingham grade, HER2 IHC staining (2 + vs 3 +).
The associations between clinicopathologic parameters and RCB class were evaluated by univariate and multivariate logistic regression analyses. A p-value less than 0.05 was considered statistically significant.
Results
Clinicopathologic features
Among 173 cases, 87 (50.3%) had pCR, and 86 (49.7%) were non-pCR. Table 1 summarizes the clinicopathologic features of 173 cases. The mean age was 53.2 and 54.6 years and the mean preoperative tumor size was 3.6 and 3.6 cm in the pCR and non-pCR group, respectively. Seventy five (55.2%) of 126 cases with HER2 IHC 3 + achieved pCR, whereas 9 (24.3%) of 37 cases with IHC 2 + but positive FISH results achieved pCR. Ten cases were FISH positive but missed IHC results and tissue blocks were not available for HER2 IHC study. The mean HER2 ratio and copy # were significantly higher in the pCR vs non-pCR group (7.4 vs 4.6 and 19.0 vs 11.9, respectively).
PD-L1 expression and immune cells in HER2 + breast cancer
PD-L1 expression was low in HER2+ breast cancer. Only 5 (7.9%) of 63 cases were positive for PD-L1 staining in tumor cells. Five were positive for stromal PD-L1 expression, including 4 cases that were positive for both tumor cell and stromal PD-L1 expression. In total, 6 (9.2%) cases expressed PD-L1 in either tumor cells or stroma. CD8+ cells were identified in 67/67 cases and FOXP3+ cells were in 66/66 cases. The mean number of CD8+ cells was similar in the pCR and non-pCR group (469.2 vs 472.5). The mean number of FOXP3+ cells was higher in the pCR group (249 vs 156.6). We were able to evaluate TILs in 111 cases, and only 11 (9.9%) showed TIL hot spot. Four cases and one case did not have enough tissues on deeper section for PD-L1 and FOXP3 IHC evaluation, respectively.
Correlation of clinicopathologic parameters with pCR
In the univariate analysis, low ER and PR expression, high Ki-67, HER2 IHC 3 + , high HER2 ratio and copy #, and high counts of FOXP3+ cells significantly correlated with pCR rate (Table 2). TIL hotspot has a non-significant correlation with pCR rate (p = 0.096). In multivariate analysis, Ki-67 index was the only variable significantly associated with pCR after controlling for other covariates (Table 3).
Discussion
In a recent publication, our group showed that HER2 IHC 3 + , high Ki-67, and high HER2 copy number were significantly associated with tumor response to neoadjuvant HER2-directed chemotherapy [19]. This is consistent with our understanding of tumor response to therapies. Cancers with strong HER2 overexpression are more likely to respond to HER2-directed therapy and the most rapidly proliferating tumor cells with high Ki-67 are sensitive to chemotherapy. These are useful predictors of response and can provide useful information as predicting which patients are likely to achieve pCR and which patients are in higher risk with residual disease and in need of more intense adjuvant therapy.
In this study, we aimed to clarify the role of the tumor immune microenvironment and to understand whether TILs, PD-L1 expression, or specific subtype of lymphocytes correlated with pCR rate and could be additional biomarkers in predicting response to therapy. Studies have shown breast cancer with extensive TIL had better prognosis even in traditionally higher-risk breast cancer subtypes [20,21,22,23,24]. Other studies have shown that heavy tumor lymphocyte infiltration is relatively rare in HER2+ breast cancer,however, along the lines of 16% [25]. In some studies, patients with more TILs have been shown to have better outcomes in terms of response to neoadjuvant therapy, event-free survival, and even overall survival [26, 27]. However, in other studies, TIL was not associated with tumor response to therapy in HER2+ breast cancer [16, 17]. In our study, 9.9% of cases showed TIL hot spot and there was a non-significant correlation between TIL hot spot and pCR (p = 0.096), indicating not only TIL but the location of TIL may correlate with tumor response. It is possible that a stronger correlation between TIL and pCR could be seen in a larger study. The non-significant correlation may also indicate HER2+ breast cancer is not as immunogenic as triple-negative breast cancer. At least one study showed TIL during treatment might predictive of pCR rate, indicating the importance of dynamic changes of TIL in tumor response to therapy and prognosis [16].
Some studies showed a positive correlation between cytotoxic CD8+ T cells and higher pCR rate and better prognosis in HER2+ breast cancer [28, 29], while other studies failed to demonstrate such correlations [30, 31]. One study showed low CD8+ cell count was associated with inferior response to lapatinib to the response to trastuzumab in metastatic HER2+ cancer although they did not see overall correlation between CD8+ T cell count and prognosis [31]. We did not find a significant correlation between CD8+ T cells and pCR rate in this study and the mean of CD8+ cells was similar between the pCR group and non-pCR group. FOXP3+ regulatory T cells has been mostly studied in triple-negative breast cancer. Few studies examined the role of FOXP3+ T cells in HER2+ cancer and the results are conflicting. One study showed FOXP3+ cells were associated with poor response to therapy and prognosis in HER2+ breast cancer [32]. Other studies did not demonstrate such correlation [33, 34]. This discrepancy could be due to difference in patient population, treatment regimens and methods in evaluation of CD8+ and FOXP3+ cells. It has been shown the concordance of inter-pathologist evaluations of CD8+ and FOXP3+ cells is low [35]. In this study, we found a positive correlation between FOXP3+ cell count and pCR rate in univariate analysis but not in multivariate analysis. One study showed a positive correlation between FOXP3+ cell count and Ki-67 level in breast cancer [34]. However, we did not see such correlation (data not shown). In the multivariate analysis, high Ki-67 level was the only factor associated with pCR rate. The precise role of FOXP3+ in tumor response in HER2+ breast cancer warrants further studies.
Higher PD-L1 expression in primary breast cancer is generally associated with better outcomes, although most of the work was conducted in triple-negative breast cancer [36, 37]. A study with a cohort of 216 cases of locally advanced HER2+ breast cancer showed an 18% frequency of PD-L1 expression and an association of PD-L1 expression with higher TIL level and pCR rate [38]. In our study, we did not see a significant correlation between PD-L1 staining and pCR rate but 9.2% of cases were positive for PD-L1 staining, indicating a possible role of immunotherapy in a subset of HER2+ breast cancer. The PD-L1 antibody we used (clone E1L3N, Cell Signaling) is different from the Ventana SP142 clone used in the IMpassion130 clinical trial in triple-negative breast cancer [39, 40]. Lawson et al. showed that although the PD-L1 epitope the E1L3N antibody binds to overlaps with the epitope the SP142 binds to, these epitopes are not identical [41]. Compared with the E1L3N clone and other FDA approved PD-L1 antibodies, the SP142 antibody has been consistently shown to detect fewer PD-L1 positive cells [42,43,44]. Other clinical trials investigating the efficacy of immune checkpoint inhibitors on triple-negative breast cancer, such as the KEYNOTE-086 [45, 46], KEYNOTE-552 [47], TONIC [48] and GeparNuevo trial [49], used different PD-L1 antibodies and positive cutoffs. These antibodies recognize different epitope of PD-L1 and may show different PD-L1 staining results [41]. Research is needed to examine which PD-L1 antibody best correlates with prognosis and tumor response in HER2+ breast cancer.
The limitation of this study includes the relatively small case number. Some of the cases were consultation cases and we did not have tissue blocks for IHC studies. Larger studies may show more significant correlation between TIL and FOXP3+ cells and pCR rate in HER2+ breast cancer. Nevertheless, we performed extensive evaluations of the clinicopathological features and immune cell profiles in this study.
More work needs to be done to determine the relevance of PD-L1 expression, TILs and specific subtypes of immune cell in HER2+ breast cancer. Such information will be critical in our understanding how we can better predict response to modern therapies and how we might use this information to design clinical trials and further optimize therapies. There are several recent trials in advanced stage HER2+ breast cancer suggesting that immunotherapy may be able to enhance the response to HER2-directed therapy in patients with PD-L1 positive disease and high TIL expression. The randomized phase II KATE2 study evaluated the addition of atezolizumab to trastuzumab emtansine in patients with advanced HER2+ breast cancer who had already received trastuzumab- and taxane-based therapy. In the atezolizumab + trastuzumab emtansine group, patients with PD-L1 + disease had longer progression-free survival (PFS) compared to those with PD-L1-negative disease, as did those with TILs ≥ 5% compared to those with TILs < 5%; whereas in the trastuzumab emtansine alone group, patients with PD-L1-negative cancer and TIL < 5% did better [50]. The PANACEA study looked at pembrolizumab in combination with trastuzumab in patients with HER2+ metastatic breast cancer that had progressed on trastuzumab treatment. In this study, 15% of the 46 PD-L1-positive patients achieved an objective response, while there were no objective responders in the PD-L1-negative subgroup [51]. These trials clearly demonstrates the importance of evaluation of PD-L1 and immune cells in patients with HER2+ breast cancer. Immunotherapy might contribute meaningfully to better outcomes in patients with PD-L1 expression or high TILs in the tumor.
Conclusion
Our findings demonstrate that high Ki-67 is a strong predictor of pCR in HER2+ breast cancer. TIL and FOXP3+ T cells may play an important role in tumor response. A subset of HER2+ breast cancer expresses PD-L1. Larger studies with HER2+ breast cancers will be needed to continue to elucidate the role of PD-L1, TILs, and other markers of immunogenicity as predictors of response to neoadjuvant chemotherapy and as risk stratification in HER2+ breast cancer.
References
Cronin KA, Lake AJ, Scott S (2018) Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 124:2785–2800
Arciero CA, Guo Y, Jiang R et al (2019) ER(+)/HER2(+) breast cancer has different metastatic patterns and better survival than ER(-)/HER2(+) breast cancer. Clin Breast Cancer 19:236–245
Li X, Wei B, Sonmez C et al (2017) High tumor budding count is associated with adverse clinicopathologic features and poor prognosis in breast carcinoma. Hum Pathol 66:222–229
Li X, Zhang Y, Meisel J et al (2018) Validation of the newly proposed American Joint Committee on Cancer (AJCC) breast cancer prognostic staging group and proposing a new staging system using the National Cancer Database. Breast Cancer Res Treat 171:303–313
Meisel J, Zhang C, Neely C et al (2018) Evaluation of prognosis in hormone receptor-positive/HER2-negative and lymph node-negative breast cancer with low oncotype DX recurrence score. Clin Breast Cancer 18:347–352
Li X, Oprea-Ilies GM, Krishnamurti U (2017) New developments in breast cancer and their impact on daily practice in pathology. Arch Pathol Lab Med 141:490–498
Li X, Yang J, Krishnamurti U et al (2017) Hormone receptor-positive breast cancer has a worse prognosis in male than in female patients. Clin Breast Cancer 17:356–366
Li X, Yang J, Peng L et al (2017) Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat 161:279–287
Slamon D, Pegram M (2001) Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials. Semin Oncol 28:13–19
Schneeweiss A, Chia S, Hickish T et al (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24:2278–2284
Gianni L, Pienkowski T, Im YH et al (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13:25–32
Symmans WF, Wei C, Gould R et al (2017) Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol 35:1049–1060
von Minckwitz G, Huang CS, Mano MS et al (2019) Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 380:617–628
Denkert C, von Minckwitz G, Brase JC et al (2015) Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 33:983–991
Salgado R, Denkert C, Campbell C et al (2015) Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol 1:448–454
Nuciforo P, Pascual T, Cortes J et al (2018) A predictive model of pathologic response based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL) in HER2-positive breast cancer treated with chemo-free dual HER2 blockade. Ann Oncol 29:170–177
Llombart-Cussac A, Cortes J, Pare L et al (2017) HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol 18:545–554
Wolff AC, Hammond MEH, Allison KH et al (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med 142:1364–1382
Meisel JL, Zhao J, Suo A et al (2020) Clinicopathologic factors associated with response to neoadjuvant anti-HER2-directed chemotherapy in HER2-positive breast cancer. Clin Breast Cancer 20(1):19–24
Afghahi A, Purington N, Han SS (2018) Higher absolute lymphocyte counts predict lower mortality from early-stage triple-negative breast cancer. Clin Cancer 24:2851–2858
Curtis CN, West RB, Horst K et al (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. Clin Cancer Res 32:2959–2966
Loi S, Drubay D, Adams S et al (2019) Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol 37:559–569
Krishnamurti U, Wetherilt CS, Yang J et al (2017) Tumor-infiltrating lymphocytes are significantly associated with better overall survival and disease-free survival in triple-negative but not estrogen receptor-positive breast cancers. Hum Pathol 64:7–12
Li XB, Krishnamurti U, Bhattarai S et al (2016) Biomarkers predicting pathologic complete response to neoadjuvant chemotherapy in breast cancer. Am J Clin Pathol 145:871–878
Stanton SE, Disis ML (2016) Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer 4:59
Perez EA, Ballman KV, Tenner KS et al (2016) Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol 2:56–64
Loi S, Michiels S, Salgado R et al (2014) Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 25:1544–1550
Kurozumi S, Inoue K, Matsumoto H et al (2019) Clinicopathological values of PD-L1 expression in HER2-positive breast cancer. Sci Rep 9:16662
Peng GL, Li L, Guo YW et al (2019) CD8(+) cytotoxic and FoxP3(+) regulatory T lymphocytes serve as prognostic factors in breast cancer. Am J Transl Res 11:5039–5053
Lee KH, Kim EY, Yun JS et al (2018) The prognostic and predictive value of tumor-infiltrating lymphocytes and hematologic parameters in patients with breast cancer. BMC Cancer 18:938
Liu S, Chen B, Burugu S et al (2017) Role of cytotoxic tumor-infiltrating lymphocytes in predicting outcomes in metastatic HER2-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol 3:e172085
Takada K, Kashiwagi S (2018) Use of the tumor-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to combination therapy with pertuzumab, trastuzumab, and docetaxel for advanced HER2-positive breast cancer. J Transl Med 16:86
De Angelis C, Nagi C, Hoyt CC et al (2020) Evaluation of the predictive role of tumor immune infiltrate in patients with HER2-positive breast cancer treated with neoadjuvant anti-HER2 therapy without chemotherapy. Clin Cancer 26:738–745
Glajcar A, Szpor J, Hodorowicz-Zaniewska D et al (2019) The composition of T cell infiltrates varies in primary invasive breast cancer of different molecular subtypes as well as according to tumor size and nodal status. Virchows Arch 475:13–23
Nederlof I, De Bortoli D, Bareche Y et al (2019) Comprehensive evaluation of methods to assess overall and cell-specific immune infiltrates in breast cancer. Breast Cancer 21:151
Li X, Wetherilt CS, Krishnamurti U et al (2016) Stromal PD-L1 expression is associated with better disease-free survival in triple-negative breast cancer. Am J Clin Pathol 146:496–502
Zhang L, Wang XI, Ding J et al (2019) The predictive and prognostic value of Foxp3+/CD25+ regulatory T cells and PD-L1 expression in triple negative breast cancer. Ann Diagn Pathol 40:143–151
Hou Y, Nitta H, Wei L et al (2018) PD-L1 expression and CD8-positive T cells are associated with favorable survival in HER2-positive invasive breast cancer. Breast J 24:911–919
Schmid P, Adams S, Rugo HS et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108–2121
Schmid P, Rugo HS, Adams S et al (2020) Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21:44–59
Lawson NL, Dix CI, Scorer PW et al (2020) Mapping the binding sites of antibodies utilized in programmed cell death ligand-1 predictive immunohistochemical assays for use with immuno-oncology therapies. Mod Pathol 33:518–530
Martinez-Morilla S, McGuire J, Gaule P et al (2020) Quantitative assessment of PD-L1 as an analyte in immunohistochemistry diagnostic assays using a standardized cell line tissue microarray. Lab Invest 100:4–15
Hirsch FR, McElhinny A, Stanforth D et al (2017) PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 12:208–222
Tsao MS, Kerr KM, Kockx M et al (2018) PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol 13:1302–1311
Adams S, Schmid P, Rugo HS et al (2019) Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 30:397–404
Adams S, Loi S, Toppmeyer D et al (2019) Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 30:405–411
Schmid P, Cortes J, Pusztai L et al (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382:810–821
Voorwerk L, Slagter M, Horlings HM et al (2019) Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med 25:920–928
Loibl S, Untch M, Burchardi N et al (2019) A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 30:1279–1288
Emens L, Esteva F, Beresford M et al (2019) Abstract PD3–01: Results from KATE2, a randomized phase 2 study of atezolizumab (atezo)+trastuzumab emtansine (T-DM1) vs placebo (pbo)+T-DM1 in previously treated HER2+ advanced breast cancer (BC). Cancer Res 79:PD3-01-PD3-01
Loi S, Giobbie-Hurder A, Gombos A et al (2019) Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b–2 trial. Lancet Oncol 20:371–382
Funding
Dr. Jing Zhao received research grant from National Natural Science Foundation of China (NSFC 81602320).
Author information
Authors and Affiliations
Contributions
Conception and design: XL; Analysis and interpretation of data: JZ, YG, XL; Manuscript drafting and reviewing: All authors; XL is responsible for the overall content.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Jane Meisel has been advisor for Puma and Pfizer and received research grants from Seattle Genetics, Pfizer and Lilly. Other authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, J., Meisel, J., Guo, Y. et al. Evaluation of PD-L1, tumor-infiltrating lymphocytes, and CD8+ and FOXP3+ immune cells in HER2-positive breast cancer treated with neoadjuvant therapies. Breast Cancer Res Treat 183, 599–606 (2020). https://doi.org/10.1007/s10549-020-05819-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05819-8