Abstract
Background
Breakthrough progress has been made in Cyclin-Dependent kinase 4 and 6 (CDK4/6) inhibitors when combined with endocrine therapy (ET) for hormone receptor-positive (HR+), HER2-negative (HER2−) advanced breast cancer (ABC). Though significant improvements of progression-free survival (PFS) for CDK4/6 inhibitors were demonstrated, however, the results of overall survival (OS) profile were not consistent. This study is conducted to further evaluate the efficacy and safety of CDK4/6 inhibitors for HR+ /HER2− ABC, and explore the prefer population through subgroup analysis.
Method
We identified relevant randomized controlled trials that compared CDK4/6 inhibitors plus ET to ET alone in HR+ /HER2− ABC. We calculated the hazard ratios (HRs) for PFS and OS, and risk ratios (RRs) for objective response rate (ORR), clinical benefit rate (CBR), adverse events (AEs). Statistical analysis was performed with the random-effects model.
Result
Eight trials and 4580 patients were included in this meta-analysis. Compared to ET alone, CDK4/6 inhibitors plus ET not only produced a significantly longer PFS (HR = 0.55, 95% confidence interval [CI] 0.50–0.59, p < 0.00001), but also manifested an extension of OS (HR = 0.79, 95% CI 0.67–0.93, p = 0.004) for HR+ /HER2− ABC. Similarly, the benefit was also manifested in ORR (RR = 1.47, 95% CI 1.30–1.67, p < 0.00001) and CBR (RR = 1.20, 95% CI 1.12–1.30, p < 0.00001). The improvements of PFS were observed in the combined treatment group as both the first-line (HR = 0.56) and the second-line therapy (HR = 0.53), and irrespective of menopausal status, the presence of visceral metastasis, previous treatment with chemotherapy, their race or age. Nevertheless, more hematologic and gastrointestinal adverse events were observed with CDK4/6 inhibitors. The most common Grade 3–4 AEs is neutropenia (RR 31.95).
Conclusion
Significant advantages of PFS and OS were observed for CDK4/6 inhibitors in HR+/HER2− ABC. Furthermore, the benefit of PFS was across all subgroups. Though associated with an increased occurrence of AEs, most of which are reversible, manageable, and acceptable. Therefore, CDK4/6 inhibitors could be recommended as a preferred options for patients with HR+ /HER2− ABC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 75% of breast cancer cases are diagnosed as hormone receptor positive [1,2,3]. Endocrine therapy (ET) is the preferred option for women with hormone receptor positive (HR+), human epidermal growth factor receptor-2 negative (HER2−) breast cancer [4]. Though obvious benefits are associated with ET, about 1/4 of patients suffer from recurrent or metastatic breast cancer due to therapeutic resistance [5,6,7]. Recent studies have investigated Cyclin-dependent kinase 4 and 6 (CDK 4/6) inhibitors as an effective treatment for endocrine-resistant breast cancer [8].
Cell cycle progression is strictly regulated by a wide range of pathways including the cyclin-dependent kinases (CDK)—retinoblastoma (RB)—E2F pathway [9, 10]. The complexes of cyclin D1 and CDK4/6 mediate the phosphorylation of the RB protein, releasing E2F transcription factors from the transcriptionally repressive Rb–E2F complex. The released E2F transcription factors are free to recruit genes inducing the promotion of cell cycle progression from G1 to S phase and allowing DNA replication [9, 10]. The CDK 4/6 inhibitors regulate cell cycle progression at the G1-S checkpoint by targeting cyclin-dependent kinases 4 and 6 and have emerged as promising candidates for cancer treatment due to the importance of CDK4/6 activity in cancer cells. The dysregulated CDK-RB-E2F pathways are connected with endocrine-resistance in hormone receptor-positive breast cancers [11, 12].
Both preclinical and preliminary clinical studies suggested that CDK4/6 inhibitors may play an important role in endocrine-resistant breast cancers [10, 12,13,14,15]. Several trials have demonstrated that CDK4/6 inhibitors are associated with prolongation of progression-free survival (PFS). However, there are not consistent on overall survival (OS) profit since the benefits of OS were not observed in all trials. Furthermore, treatment-related high grade (grade 3 and grade 4) adverse events (AEs) have been frequently reported with CDK4/6 inhibitors. The monitoring of complete blood count is needed for palbociclib, ribociclib, and abemaciclib [16,17,18,19], and liver function test is required for ribociclib and abemaciclib, which might be troublesome in the clinical use [17, 18]. Recently, CDK4/6 inhibitor-related pneumonitis has warned by the Food and Drug Administration (FDA) [20].
Thus, we performed this systematic review and meta-analysis to precisely evaluate the efficiency and safety of selective CDK4/6 inhibitors when added standard endocrine agents as compared to standard endocrine agents alone for treatment of women with HR+ /HER2− ABC.
Methods
Search strategy
Searches were performed for all published and unpublished randomized controlled trials in English. We searched the following databases from Jan 2008 up to April 2019: PUBMED, MEDLINE, EMBASE, and The Cochrane Central Register of Controlled Trials. The last search was performed on April 8, 2019 with the search terms including “advanced breast neoplasm,” “cyclin-dependent kinase inhibitor,” “palbociclib,” “ribociclib,” “abemaciclib,” “endocrine therapy.” We also searched the following conference proceedings for relevant abstracts: ASCO, SABCS, ESMO, and NCCN from Jan 2008 up to April 2019 (Supplemental Table 1). In cases of reports from the same trial, the most recent results with longer follow-up were included.
Inclusion and exclusion criteria
To be included, studies had to be prospective randomized controlled, phase II or III studies that examined CDK4/6 inhibitors plus standard ET in comparison to ET alone in women of any menopausal status who were 18 years old or older with HR+/HER2− ABC. The dose of CDK4/6 inhibitors was allowed as FDA approved dose regimens (palbociclib was administered 125 mg oral once daily for 3 weeks, followed by a week off in a 28-day cycle; ribociclib was administered 600 mg oral once daily for 3 weeks, followed by a week off in a 28-day cycle; abemaciclib was administered 200–150 mg oral twice daily). We excluded reviews, letters, comments, lectures, case reports, and non-prospective studies (retrospective analysis).
Data extraction
Two review authors (JL and FMF) independently decided on the eligibility of all abstracts and potentially eligible full-text articles. Two review authors (JL and FMF) independently extracted data from the included studies using standard data extraction forms. Data extracted included study design (the phase of trials, the number of participants), treatment arms (interventions and controls), participants (races, average Age, menopause status, previous treatment, site of tumor metastasis, Eastern Cooperative Oncology Group performance status), setting, follow-up, and sources of funding. If a study was reported in more than one publication, we extracted outcome data from the final or updated version of the study.
Assessment of risk of bias
Two independent review authors (JL and FMF) assessed the risk of bias for all eligible articles using The Cochrane Collaboration's 'Risk of bias' assessment tool [21]. Any disagreements were resolved by consensus and, if required, by consulting a third author (LWY).
Assessment of reporting biases
We performed a test for funnel plot asymmetry to estimate effects from the presence of a small-study in the primary outcomes of at least ten studies in the meta-analysis [22].
Types of outcomes
The primary outcome was progression-free survival (PFS), defined as the time from randomization to disease progression or death during the study.
The secondary outcomes included clinical benefit rate (CBR, defined as a confirmed complete response, a partial response, or stable disease for 24 weeks), objective response rate (ORR, defined as a confirmed complete response or partial response), overall survival (OS, defined as the time from the date randomized to death during the study), and toxicity that recorded the occurrence of all grades of AEs and grade 3 or 4 AEs (as graded by the National Cancer Institute Common Terminology Criteria version 4.0), including three hematologic toxicities (neutropenia, leucopenia, and anemia) and four non-hematologic toxicities (diarrhea, fatigue, nausea, and arthralgia).
Subgroup analysis
We preset the subgroup analyses of primary outcomes for the following subgroups:
Comparison among different interventions: palbociclib vs. Ribociclib vs. abemaciclib.
Comparison between different lines of therapy for ABC: first-line therapy (defined as newly diagnosed ABC with no systemic therapy and relapse > 12 months from completion of (neo) adjuvant endocrine therapy with no treatment for advanced or metastatic disease) vs. second-line therapy (defined as relapse on or within 12 months from completion of (neo)adjuvant endocrine therapy with no treatment for advanced or metastatic disease and disease progressed after one line of endocrine therapy for advanced disease).
Comparison between different menopausal status: premenopausal or perimenopausal women vs. postmenopausal women.
Comparison between patients with visceral metastases vs. non-visceral metastases.
Comparison among different sequences of chemotherapy: previous chemotherapy for ABC vs. no previous chemotherapy for ABC (no previous chemotherapy or chemotherapy as (neo)adjuvant therapy).
Comparison between different races: Asian vs. non-Asian.
Comparison between different age groups: age < 65 years old vs. age ≥ 65 years old.
Statistical methods
The treatment effect was evaluated as hazard ratios (HRs) with corresponding 95% confidence interval (95% CI) for time-to-event outcomes (PFS, OS) and risk ratios (RRs) with 95% CI for dichotomous outcomes (ORR, CBR, and AEs). Due to the three interventions (palbociclib, ribociclib, and abemaciclib) and inclusion of some studies with different events, a random effect model was used in this analysis. The data analysis was calculated by Review Manager analysis software, version 5.1.0 and the results illustrated by forest plots [21]. All statistical tests were two sided with statistical significance defined as p ≤ 0.05. Statistical heterogeneity was assessed by calculating I2 statistic, and I2 value of 0–40% indicate no heterogeneity, 30–60% indicate moderate heterogeneity, 50–90% indicate substantial heterogeneity, and 75% to 100% indicate considerable heterogeneity [21].
Results
Study selection
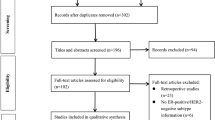
From 2294 records identified by the initial database search, 2248 studies were excluded after screening the title and abstract. Thirty-three more studies were excluded after full text and conference abstract review. The remaining 14 studies, including eight different trials, were eligible for this review comparing CDK4/6 inhibitors plus standard endocrine agents to standard endocrine agents alone for treatment of women with HR+ /HER2− ABC [23,24,25,26,27,28,29,30,31,32,33,34,35,36]. The flow diagram of the study selection process is shown in Fig. 1.
Design of studies and participants
In the eight randomized control trials, a total 4580 women were enrolled. Three trials investigated palbociclib (involved 1352 patients) [23,24,25], three investigated ribociclib (involved 2066 patients) [26,27,28], and two investigated abemaciclib (involved 1162 patients) [29, 30]. Two trials enrolled patients receiving treatment in the first-line setting for advanced breast cancer [26, 30], two trials was in the second-line setting [25, 29], and four trials both in the first-line and the second-line setting [23, 24, 27, 28]. Five trials used AI as a combination treatment of CDK4/6 inhibitors [23, 24, 26, 28, 30], three trials used Fulvestrant as endocrine therapy [25, 27, 29]. Five studies enrolled only postmenopausal women [23, 24, 26, 27, 30], one study enrolled premenopausal and perimenopausal women [28], and two studies enrolled women with any menopausal status [25, 29]. Two trials allowed previous chemotherapy for advanced breast cancer [25, 28]. Characteristics of included studies was show in Supplementary Table 2.
Progression-free Survival (PFS)
A total of 4580 women were involved in the analysis and 1824 PFS events occurred. This meta-analysis revealed that PFS significantly improved in the CDK4/6 inhibitors containing groups (HR 0.55, 95% CI 0.50–0.59, p < 0.00001, Fig. 2) with no heterogeneity regarding to this outcome (I2 = 0%). The HRs significantly favored the CDK4/6 inhibitors containing groups over the endocrine therapy alone groups in both the first-line setting (HR 0.56, 95% CI 0.49–0.63, p < 0.00001) and the second-line setting (HR 0.53, 95% CI 0.47–0.60, p < 0.00001, Fig. 3). The effects of the CDK4/6 inhibitors were consistent across all menopause status, HR = 0.52 for pre-/perimenopausal women and 0.55 for postmenopausal women (Supplemental Fig. 1). The duration of PFS was notably longer in the CDK4/6 inhibitors containing groups regardless of the presence or absence of visceral metastasis, with the HR of 0.55 for visceral metastasis and 0.54 for non-visceral metastasis (Supplemental Fig. 2). Two studies allowed prior chemotherapy for ABC with the HR 0.47, just the same as in a no prior chemotherapy setting (Fig. 4). Whether patients are Asian or non-Asian, younger than 65 years old or older than 65 years old, they had longer PFS in the CDK inhibitors contain treatment groups in comparison to those in the endocrine therapy alone group (Supplemental Figs. 3, 4).
Overall survival (OS)
Overall survival data were reported in three enrolled studies [34,35,36]. Patients in the CDK4/6 inhibitors containing group were observed to have a significantly longer overall survival than those in the ET alone group with an HR = 0.79, 95% CI 0.67–0.93, and p = 0.004 (Fig. 5).
Objective response rate (ORR) and Clinical benefit rate (CBR)
The pooled data suggested a significant increase in both ORR and CBR in CDK4/6 inhibitors containing treatment groups compared to the control groups, with the RR of 1.47 for ORR (95% CI 1.30–1.67, and p < 0.00001, Fig. 6) and 1.20 for CBR (95% CI 1.12–1.30, and p < 0.00001, Supplemental Fig. 7). In the first-line setting we found that the RR of ORR using CDK4/6 inhibitors was better than in the ET alone group, where RR = 1.44, 95% CI 1.23–1.69, and p < 0.00001. The improvement was also observed in the second-line setting with RR of 2.19, 95% CI 1.66–2.89, and p < 0.00001 (Supplemental Fig. 5). We also observed the improvements of CBR in both the first-line setting (RR = 1.09) and the second-line setting (RR = 1.45) (Supplemental Fig. 8). The improvements of ORR and CBR were consistent across all investigational drugs and in any menopausal state (Supplemental Figs. 6, 9).
Toxicity
The pooled data of all-grade toxicities were from 4555 participants in all eight studies. All-grade neutropenia is the most commonly observed AEs in CDK4/6 intervention arms (RR 14.24, 95% CI 10.91–18.59, Supplemental Fig. 10). Similarly, all-grade leucopenia and anemia were recorded more in CDK4/6 inhibitor containing regiments (RR was 10.24 for leucopenia and 3.52 for anemia, Supplemental Fig. 11–12). For all-grade non-hematologic toxicity, the RR were 1.71 (95% CI 1.23–2.37) for diarrhea, 1.24 (95% CI 1.08–1.41) for fatigue, 1.63 (95% CI 1.44–1.84) for nausea, and 0.98 (95% CI 0.87–1.09) for arthralgia (Supplemental Figs. 13–16).
The grades 3 and 4 (G3-4) neutropenia were increased in intervention arms than control arms, the RR was 31.95 (95% CI 17.75–57.50) with substantial heterogeneity among different interventions (I2 = 58.8%, Fig. 7). There are 1.3% of patients recorded to occur febrile neutropenia in CDK4/6 inhibitors intervention arms, and 0.2% patients in control arms. The absolute risk was 1.1%. No cases of death due to neutropenic fever were reported. Similarly, there are more records about the G3-4 leucopenia and anemia in the CDK4/6 inhibitors intervention arms with an RR 22.08 for leucopenia and 2.24 for anemia (Supplemental Figs. 17, 18). The RR of G3-4 diarrhea was 2.60 (95% CI 0.94–7.16, p = 0.06) with a substantial heterogeneity among the eight studies (I2 = 57%, Supplemental Fig. 19). In the subgroup analysis of different interventions, the incidence of G3-4 diarrhea was significantly higher in patients receiving abemaciclib (RR 12.62, 95% CI 3.48–45.82). Levels G3-4 fatigue and nausea are related to CDK4/6 inhibitor use with an RR 3.14 and 1.33, respectively (Supplemental Fig. 20, 21).
Risk of bias
The PALOMA-1 was an open-label trial while the other enrolled studies are all double-blind trials with a low risk of bias for the most outcome assessments. The detail description of the risk of bias results is provided in Table 1. The Interventions Reporting bias were performed for primary outcomes PFS using funnel plots and the plots were symmetrical, the degree of asymmetry were performed by Begg’s test and no evidence of publication bias was observed (Supplemental Fig. 22).
Discussion
This meta-analysis demonstrated that CDK 4/6 inhibitors plus standard ET produced a remarkable improvement of PFS and OS in women with HR+ /HER2− ABC, moreover, the benefits were seen in all subgroups. There was an increased occurrence of neutropenia, leucopenia, and diarrhea associated with CDK4/6 inhibitors.
To the best of our knowledge, this is the first meta-analysis assessing patients receiving CDK4/6 inhibitors as first-line or second-line therapy for advanced breast cancer. The statistical analysis showed significantly longer PFS in the CDK4/6 inhibitors treatment groups in both first- and second-line therapy groups, as well as improved ORR and CBR. The results demonstrated the low magnitude of improvement of CBR in first-line therapy, but given that advanced breast progression usually occurs in more than 24 weeks when the endocrine therapy is used alone in the first-line treatment [37, 38], the results should be interpreted with deliberation. In previous meta-analyses, PFS results favored the use of fulvestrant versus other endocrine therapies for HR+ /HER2− ABC patients, but the difference did not reach statistical significance [39, 40]. In addition to subgroup analyses, there were no statistically significant improvements in PFS regardless of fulvestrant as the first-line or the second-line therapy [39]. In our current meta-analysis, approximately 80% of patients had received 500 mg fulvestrant as their second-line therapy and the PFS was significantly longer in the CDK4/6 inhibitors treatment group. Therefore, we have reason to believe that the introduction of CDK4/6 inhibitors to fulvestrant can improve PFS.
The FIRST trial demonstrated that fulvestrant at 500 mg reduced by 30% the risk of death (HR 0.70, 95% CI 0.50–0.98, p = 0.04) when compared with anastrozole in the first-line setting for HR+ ABC. It's worth noting that either the improvement of time to progression (TTP) or OS were limited in patients who had previously received chemotherapy or endocrine in the subgroup analysis [41]. Furthermore, the OS analysis was not planned and about 17% of patients did not participate in OS follow-up [41]. Additionally, differences in the OS were not statistically significant between fulvestrant and aromatase inhibitors in a previous meta-analysis (HR 0.89, 95% CI 0.70–1.13) [40]. Buparlisib, an oral pan-PI3K inhibitor, combined with fulvestrant resulted in a 13% relative risk reduction versus placebo plus fulvestrant. However, the difference was not statistical significant (HR 0.87, 95% CI 0.74–1.02) [42]. It needs to be highlighted that no only significant improvements of PFS but also the substantial advantage of OS were observed in the CDK4/6 inhibitors contained group in our meta-analysis. The CDK4/6 inhibitors related to a 21% lower risk of death (Fig. 5). Overall survival differences are rarely seen in endocrine therapy, and the OS benefits of CDK4/6 inhibitors in this meta-analysis are an encouraging outcome. However, only three of eight enrolled studies contributed to the OS statistics, the results of OS analysis after longer follow-up from other enrolled trails are expected to further confirm the better effect of CDK4/6 inhibitors.
Though endocrine therapy is recommended as first-line therapy for patients with advanced HR+ /HER2− breast cancer, in the real-world more than 30% of patients receive chemotherapy as first-line treatment for advanced or metastatic disease [43,44,45]. Patients who had initial systemic treatment with chemotherapy were often younger, with a pre-/perimenopausal status, or had visceral metastases. Our meta-analysis showed meaningful PFS improvements from adding CDK4/6 inhibitors in all the above subgroups (Supplemental Figs. 1, 2, and 4). Besides, a PFS benefit was seen in those whose disease progressed after prior chemotherapy for ABC (Fig. 4). Noticeably, the KCSG-BR 15–10 trail compared CDK4/6 inhibitors with chemotherapy and demonstrated that palbociclib plus ET result in 7.7 months longer PFS versus capecitabine alone in premenopausal women with HR+ metastatic breast cancer (PFS: 19.0 months vs. 11.3 months, HR = 0.643, p = 0.0493) [46]. This finding was consistent with a previous network meta-analysis [47]. Furthermore, CDK 4/6 inhibitors have lower toxicity than chemotherapy [48], therefore, the CDK4/6 inhibitors could be a preferred choice instead of chemotherapy in patients without visceral crisis.
Neutropenia was the most common toxicity reported with CDK4/6 inhibitor, particular with palbociclib and ribociclib treatment (Fig. 7). Different from chemotherapy, CDK4/6 inhibitor-related neutropenia is due to cell cycle arrest and cells remain functional and can rapidly reverse growth arrest after withdrawal of CDK4/6 inhibitors [48]. Although there was a higher incidence of G3-4 neutropenia (50.43% patients) in the CDK4/6 inhibitor arms, it was not often accompanied by serious clinical outcomes. About 1.3% of patients showed febrile neutropenia and no cases of death due to neutropenic fever were reported. Even so, the monitoring of complete blood count is still necessary [16,17,18,19]. About 84.2% patients suffer from diarrhea in the abemaciclib arm. Discontinuation of the drug was reported in 17.31% (134 of 774) of patients in the abemaciclib arms, of these, 2.7% were attributed to diarrhea. However, the abemaciclib-related diarrhea could be effectively managed in most case with conventional antidiarrheal medications and dose adjustments due to the lower grade (G1-2) [29, 30, 49]. Ribociclib has an impact on the increase of the ECG QTcF interval, similar to other cancer therapies [50]. This is a dose-dependent side effect of ribociclib and most patients were able to accept continued treatment without dose interruption [26]. Nevertheless, it is important to avoid using ribociclib in patients with prolonged QT intervals [17]. No cases of Torsades de Pointes were reported in these studies [26,27,28]. Despite six patients reported with cases of Hy’s law in the ribociclib arms, the aminotransferase and bilirubin levels returned to normal in all patients after the discontinuation of ribociclib [26,27,28]. Nevertheless, the aminotransferase monitoring are recommended for patients when treated with ribociclib, so are ECG and electrolyte monitoring which was cited as a nuisance by several expert physicians and clinical researcher [50].
There are several limitations of our meta-analysis. First, the aggregate data are from published articles instead of individual patient data. Second, the inclusion of three different CDK 4/6 inhibitors (palbociclib, ribociclib, and abemaciclib) in the analysis may result in heterogeneity of statistical results. However, those interventions have no heterogeneity in terms of efficacy analysis. Third, since there is a lack of mature OS data for more than half of enrolled trials, the interpretation of results needs to be taken cautiously. There are also some strengths in our meta-analysis. This study included all newly updated data of trials comparing efficacy and safety of CDK 4/6 inhibitors added to endocrine therapy vs. endocrine therapy alone for treatment of HR+ /HER2− ABC. Moreover, we performed several subgroup analyses in order to find the target population who have better responses to CDK4/6 inhibitors, and this is the first meta-analysis assessing CDK4/6 inhibitors as first-line or second-line therapy for HR+ /HER2− ABC.
Conclusion
The CDK4/6 inhibitors (including palbociclib, abemaciclib, and ribociclib) plus standard endocrine agents prolong PFS and OS and show benefit in ORR and CBR in HR+ /HER2− ABC irrespective of the prior therapy for advanced disease, menopausal status, the existence of visceral metastases, and different races. Though followed by the increasing occurrence of neutropenia, leucopenia, and diarrhea, most of the adverse events are reversible, manageable and acceptable. Given their superior efficacy and tolerable toxicity, the CDK4/6 inhibitors could be recommended as a preferred option for the majority of patients with HR+ /HER2− ABC.
Data availability
The data supporting the conclusions of this article are included within the article and its additional file.
Abbreviations
- ABC:
-
Advanced breast cancer
- AEs:
-
Adverse effects
- AIs:
-
Aromatase inhibitors
- CBR:
-
Clinical benefit rate
- CDK4/6:
-
Cyclin-Dependent kinase 4 and 6
- CI:
-
Confidence interval
- ET:
-
Endocrine therapy
- HER2− :
-
Human epidermal growth factor receptor-2 negative
- HRs:
-
Hazard ratios
- HR+ :
-
Hormone receptor-positive
- RR:
-
Risk ratio
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
References
Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA et al (2014) US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju055
Kwan ML, Kushi LH, Weltzien E, Maring B, Kutner SE et al (2009) Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res 11(3):R31
Onitilo AA, Engel JM, Greenlee RT, Mukesh BN (2009) Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res 7(1–2):4–13
Migliaccio I, Malorni L, Hart CD, Guarducci C, Di Leo A (2015) Endocrine therapy considerations in postmenopausal patients with hormone receptor positive, human epidermal growth factor receptor type 2 negative advanced breast cancers. BMC Med 13(1):46
Osborne CK, Schiff R (2011) Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 62:233–247
Hölzel D, Eckel R, Bauerfeind I, Baier B, Beck T et al (2017) Survival of de novo stage IV breast cancer patients over three decades. J Cancer Res Clin Oncol 143(3):509–519
Ring A, Dowsett M (2004) Mechanisms of tamoxifen resistance. Endocr Relat Cancer 11(4):643–658
Murphy CG, Dickler MN (2016) Endocrine resistance in hormone-responsive breast cancer: mechanisms and therapeutic strategies. Endocr Relat Cancer 23(8):R337–R352
Johnson J, Thijssen B, McDermott U, Garnett M, Wessels LF, Bernards R (2016) Targeting the RB-E2F pathway in breast cancer. Oncogene 35(37):4829–4835
Hamilton E, Infante JR (2016) Targeting CDK4/6 in patients with cancer. Cancer Treat Rev 45:129–138
Baker SJ, Reddy EP (2012) CDK4: a key player in the cell cycle, development, and cancer. Genes Cancer 3(11–12):658–669
Finn RS, Aleshin A, Slamon DJ (2016) Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res 18(1):17
Shapiro GI (2006) Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol 24(11):1770–1783
Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M et al (2004) Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 3(11):1427–1438
Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ et al (2014) Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs 32(5):825–837
IBRANCE (2017) Prescribing information: palboci-clib. Pfizer Inc., New York
KISQALI (2017) Prescribing information: ribociclib. Novartis Pharmaceuticals Corporation, East Hanover, NJ
VERZENIO (2017) Prescribing information: abemaciclib. Eli Lilly and Company, Indianapolis, IN
Thill M, Schmidt M (2018) Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther Adv Med Oncol. 10:1758835918793326.
US Food and Drug Administration (2019) FDA warns about rare but severe lung inflammation with Ibrance, Kisqali, and Verzenio for breast cancer. https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare-severe-lung-inflammation-ibrance-kisqali-and-verzenio-breast-cancer. Accessed 13 Dec, 2019
Higgins JPT, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. www.cochrane-handbook.org
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR et al (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343:d4002
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM et al (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16(1):25–35
Finn RS, Martin M, Rugo HS, Jones S, Im SA et al (2016) Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 375(20):1925–1936
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im S-A et al (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17(4):425–439
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS et al (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375(18):1738–1748
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M et al (2018) Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 36(24):2465–2472
Tripathy D, Im S-A, Colleoni M, Franke F, Bardia A et al (2018) Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 19(7):904–915
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K et al (2017) MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35(25):2875–2884
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S et al (2017) MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35(32):3638–3646
Rugo HS, Finn RS, Dieras V, Ettl J, Lipatov O et al (2017) Palbociclib (PAL) + letrozole (LET) as first-line therapy in estrogen receptor–positive (ER+)/human epidermal growth factor receptor 2–negative (HER2−) advanced breast cancer (ABC): Efficacy and safety updates with longer follow-up across patient subgroups. San Antonio Breast Cancer Symposium 2017, Abstract P5–21–03
Spazzapan S, Conte P, Simoncini E, Campone M, Miller M, Sonke G (2017) Updated results from MONALEESA-2, a phase 3 trial of first-line ribociclib 1 letrozole in hormone receptor-positive (HR1), HER2- negative (HER2) advanced breast cancer (ABC). Annu Oncol 28(Suppl 6):vi 28.
Iwata H, Im SA, Masuda N, Im YH, Inoue K et al (2017) PALOMA-3: phase III trial of fulvestrant with or without palbociclib in premenopausal and postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer that progressed on prior endocrine therapy-safety and efficacy in Asian patients. J Glob Oncol 3(4):289–303
Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M et al (2019) Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 381(4):307–316
Finn RS, Crown J, Lang I, Boer K, Bondarenko I et al (2017) Overall survival results from the randomized phase II study of palbociclib (P) in combination with letrozole (L) vs letrozole alone for frontline treatment of ER+/HER2- advanced breast cancer (PALOMA-1; TRIO-18). J Clin Oncol 35(Suppl 15):1001
Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA et al (2018) Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 379(20):1926–1936
Mouridsen H, Gershanovich M, Sun Y, Pérez-Carrión R, Boni C et al (2001) Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 19(10):2596–2606
Robertson JFR, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L et al (2016) Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 388(10063):2997–3005
Lee CI, Goodwin A, Wilcken N (2017) Fulvestrant for hormone-sensitive metastatic breast cancer. Cochrane Database Syst Rev 1:CD011093
Wang J, Xu B, Wang W, Zhai X, Chen X (2018) Efficacy and safety of fulvestrant in postmenopausal patients with hormone receptor-positive advanced breast cancer: a systematic literature review and meta-analysis. Breast Cancer Res Treat 171(3):535–544
Ellis MJ, Llombart-Cussac A, Feltl D, Dewar JA, Jasiówka M et al (2015) Fulvestrant 500 mg Versus Anastrozole 1 mg for the First-Line Treatment of Advanced Breast Cancer: Overall Survival Analysis From the Phase II FIRST Study. J Clin Oncol 33(32):3781–3787
Campone M, Im SA, Iwata H, Clemons M, Ito Y et al (2018) Buparlisib plus fulvestrant versus placebo plus fulvestrant for postmenopausal, hormone receptor-positive, human epidermal growth factor receptor 2-negative, advanced breast cancer: overall survival results from BELLE-2. Eur J Cancer 103:147–154
Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F et al (2016) In real life, one-quarter of patients with hormone receptor-positive metastatic breast cancer receive chemotherapy as initial palliative therapy: a study of the Southeast Netherlands Breast Cancer Consortium. Ann Oncol 27(2):256–262
Twelves C, Jove M, Gombos A, Awada A (2016) Cytotoxic chemotherapy: still the mainstay of clinical practice for all subtypes metastatic breast cancer. Crit Rev Oncol Hematol 100:74–87
Caldeira R, Scazafave M (2016) Real-world treatment patterns for hormone receptor- positive, human epidermal growth factor receptor 2-negative advanced breast cancer in Europe and the United States. Oncol Ther 4(2):189–197
Park YH, Kim TY, Kim GM, Jung KH, Kang SY et al (2019) A randomized phase II study of palbociclib plus exemestane with GNRH agonist versus capecitabine in premenopausal women with hormone receptor-positive metastatic breast cancer (KCSG-BR 15–10, NCT02592746). J Clin Oncol 37(15_suppl):1007
Wilson FR, Varu A, Mitra D, Cameron C, Iyer S (2017) Systematic review and network meta-analysis comparing palbociclib with chemotherapy agents for the treatment of postmenopausal women with HR-positive and HER2-negative advanced/metastatic breast cancer. Breast Cancer Res Treat 166(1):167–177
Hu W, Sung T, Jessen BA, Thibault S, Finkelstein MB, Khan NK, Sacaan AI (2016) Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res 22(8):2000–2008
Dickler MN, Tolaney SM, Rugo HS, Cortes J, Dieras V et al (2017) MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(-) metastatic breast cancer. Clin Cancer Res 23(17):5218–5224
Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E et al (2012) Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann Oncol 23(Suppl 7):vii155–166
Funding
No funding received.
Author information
Authors and Affiliations
Contributions
JL, FMF, LWY, and CW participated in the conception, design, study evaluation for inclusion, and data interpretation. JL, FMF, and LWY participated in data extraction. JL and MH performed the statistical analysis. JL wrote the first manuscript. FMF, YXL, LWY, QM, and JXL helped in drafting the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable (no informed consent required).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, J., Fu, F., Yu, L. et al. Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: a meta-analysis of randomized clinical trials. Breast Cancer Res Treat 180, 21–32 (2020). https://doi.org/10.1007/s10549-020-05528-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05528-2