Abstract
Background
Endocrine therapy with aromatase inhibitors (AIs) is the cornerstone of adjuvant systemic treatment for postmenopausal patients with hormone receptor-positive breast cancer. It has become clear that hormone receptor-positive breast cancer carries a consistent risk of relapse up to 15 years after diagnosis. Extended duration of adjuvant AIs therapy after completing initial standard adjuvant AIs-containing therapy may prevent late recurrence and death. We performed a meta-analysis to assess the real impact of the extended adjuvant therapy with AIs.
Methods
A literature-based meta-analysis of the randomized controlled trials (RCTs) was undertaken. Relevant publications from PubMed, Embase, Cochrane Library, and abstracts from American Society of Clinical Oncology (ASCO) and San Antonio Breast Cancer (SABCS) symposia were searched. The endpoints were disease-free survival (DFS), overall survival (OS), local recurrence, distant recurrence, contralateral breast cancer, non-breast cancer-related death, and toxicity.
Results
Eight trials comprising 15,966 patients met the inclusion criteria. The pooled analysis revealed a significant improvement in DFS (RR = 0.79; 95% CI 0.68–0.91), distant recurrence (RR = 0.75; 95% CI 0.58–0.96), and contralateral breast cancer (RR = 0.53; 95% CI 0.40–0.70) in the extended AIs group. While there was not significant improvement in OS (RR = 1.00, 95% CI 0.99–1.01), non-breast cancer-related death (RR = 1.16, 95% CI 0.96–1.41), and local recurrence (RR = 0.82; 95% CI 0.64–1.06), the subgroup analysis showed that the patient with tumor size > 2 cm (HR = 0.74, RD = − 0.31, P = 0.05 vs. HR = 0.85, RD = − 0.16, P = 0.20), node positive status (HR = 0.77, RD = − 0.27, P = < 0.0001 vs. HR = 0.89, RD = −0.12, P = 0.19) and previous chemotherapy use (HR = 0.75, RD = − 0.29, P = 0.003 vs. HR = 0.91, RD = −0.10, P = 0.44) would get a greater DFS benefit with extended AIs. Longer treatment with AIs was associated with an increased risk ratio of bone pain (RR = 1.26, RD = 0.04, P = 0.003), bone fractures (RR = 1.59, RD = 0.02, P = 0.002), osteoporosis (RR = 1.53, RD = 0.07, P = 0.005), myalgia (RR = 1.26, RD = 0.04, P = 0.02), and treatment discontinuation for adverse events (RR = 1.51, RD = 0.06, P = 0.0009).
Conclusion
After initial standard AIs-containing adjuvant therapy, extended AIs therapy could further bring a DFS benefit for postmenopausal patients with early breast cancer, especially in the patients with high-risk characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Hormonal therapy is the cornerstone of adjuvant systemic treatment for patients with hormone receptor-positive breast cancer. Possible therapies include tamoxifen, aromatase inhibitors (AIs) or, in pre-menopausal patients, ovarian function suppression (OFS) achieved by surgery (oophorectomy) or by GnRH agonists [1]. Multiple large clinical trials have showed that 5 years of adjuvant endocrine therapy substantially reduces the risks of locoregional and distant recurrence, contralateral breast cancer, death from breast cancer, and death from any cause [2,3,4].
Despite the success of adjuvant endocrine therapy, still 50 % of all recurrences occur after the first 5 years [5]. In recent decades, multiple clinical trials have tested the role of extending adjuvant endocrine therapies in patients with hormone receptor-positive disease. Extended adjuvant endocrine therapy with either tamoxifen or AIs after 5 years of initial tamoxifen treatment has been shown to improve the disease-free survival (DFS) in early-stage breast cancer [6,7,8,9].
However, the EBCTCG meta-analysis has shown that the adjuvant therapy containing AIs in the first 5 years of adjuvant therapy was superior to tamoxifen monotherapy [4]. Nowadays AIs are one of the most commonly used treatments in adjuvant endocrine therapy for postmenopausal women with breast cancer [10,11,12], which has achieved good therapeutic effects. Thus, whether it is necessary to adopt extended adjuvant AIs therapy after completing AIs-containing therapy is not clear. The MA.17R is the first study to examine the role of extended AIs therapy for up to 10 years. After a median follow-up of 6.3 years, the study showed a significant reduction in DFS in favor of letrozole (HR 0.66, P = 0.01). These results provide support for consideration of extended AIs therapy in all women who have tolerated standard adjuvant AIs-containing therapy. However, the NSABP B-42 trial showed that letrozole therapy did not significantly prolong DFS in patients who had remained free of breast cancer after completing 5 years of AIs-containing therapy.
In view of the variability in results in trials of extended adjuvant AIs therapy, the true benefit of this treatment strategy is unknown. Here, we report a meta-analysis of randomized trials to assess the differences in efficacy and toxicity of the extended adjuvant AIs therapy after completing the initial standard AIs-containing therapy.
Methods
A systematic literature review of the published RCTs was performed and the meta-analysis was conducted in accordance with the Preferred Reporting for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. This meta-analysis did not require any review of protocols or registration.
Data sources and search strategy
PubMed, Embase, and Cochrane Library were used to identify all eligible trials. Keywords used were: “breast cancer”, “endocrine therapy”, “extended or continued or prolonged”, “aromatase inhibitor”. Furthermore, we searched abstracts and presentations reported from annual meetings of the American Society of Clinical Oncology (ASCO) or the San Antonio Breast Cancer Symposium (SABCS) to collect relevant unpublished studies. Lastly, all review articles and the cross referenced manuscripts from retrieved articles were screened for relevant studies.
Inclusion criteria
We included all the RCTs which met the following criteria: RCT's that were published in English, patients of any age with hormone receptor-positive early or locally advanced breast cancer, RCT’s which investigated the outcomes of extended adjuvant AIs-containing therapy in postmenopausal women who had remained free of breast cancer after completing AIs-containing therapy, assessed the DFS, OS, local recurrence, distant recurrence, contralateral breast cancer, non-breast cancer-related death, and toxicity. There was no time restriction on publication dates.
Exclusion criteria
Studies, which were not RCTs, case series, reviews, letters, editorials, non-peer reviewed studies, and duplicates were excluded from the analysis.
Data extraction
Articles were retrieved from databases using mentioned keywords. Selection of articles was done in 3 steps. The Endnote X7 Resources Management Software was used to organize, study titles and abstracts, and identify duplicates. After removing duplicates, the titles of all articles reviewed and articles that didn’t match with inclusion criteria were removed. For the next step, the abstract and the full-text of the articles were studied and checked based on inclusion criteria and study objectives. The study selection and quality assessment were done by two researchers independently, and in case of disagreement the subject was referred to a third person. Information extracted from the articles was summarized in the extraction form.
Risk of bias assessment
Review Manager 5.3 from the Cochrane Collaboration was used to assess the risk of bias in individual studies. Uniform criteria were recommended by the Cochrane Collaboration, which included six items: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other potential bias as previously used were applied in our meta-analysis.
Statistical analyses
The differences between the two groups were estimated by the pooled RR and HR along with 95% CIs. The summary RR or HR estimates were conducted using a random or fixed effect model. Subgroup analyses were performed to detect the influence of stratification factors and other baseline characteristics. Statistical heterogeneity was estimated by the I2 statistic as follows: I2 < 30% meant “low heterogeneity”; I2 between 30 and 50% denoted “moderate heterogeneity”; I2 > 50% represented “substantial heterogeneity.” A fixed effect model was used if the heterogeneity was low or moderate. Otherwise, the random effect model was reported after exploring the cause of heterogeneity. All calculations were performed by Review Manager Version 5.3 software (The Cochrane Collaboration, Oxford, UK). All tests mentioned below were 2-tailed and a P value of < .05 was considered to be statistically significant for all analyses.
Results
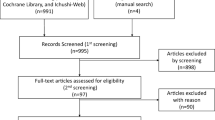
According to the search strategy established by us, 708 records were retrieved totally from PubMed, Embase, and Cochrane Library. After removing the duplicates and irrelevant records, 74 full-text articles were available for the meta-analysis. However, 66 studies were excluded after full-text article assessment, leaving a total of 8 RCTs that contained sufficient details or met the inclusion criteria (Fig. 1) [13,14,15,16,17,18,19,20]. The majority of the included studies evaluated the effects of further extension of adjuvant AIs therapy which had completed the AIs-containing therapy, and detailed treatment strategies were shown in Fig. 2. All studies’ characteristics have been summarized in Tables 1.
Disease-free survival (DFS) and overall survival (OS)
All 8 RCTs enrolling 15,966 postmenopausal patients were available for the analysis of DFS at the end of the observation period. The random effects model was used because there were high heterogeneities (I2 = 68%, P = 0.003) between these data. The pooled data showed that extended endocrine therapy with AIs improved DFS when compared to control (RR = 0.79; 95% CI 0.68–0.91; Fig. 3).
Data for OS were available in 7 trials, and OS was similar in the two groups. The fixed effects model was used for the analysis of the OS data due to the presence of low heterogeneity (I2 = 0%) between the trials. The pooled analysis revealed that extended AIs therapy did not decrease the risk of death from any cause (RR = 1.00, 95% CI 0.99–1.01; Fig. 4a), as well as non-breast cancer-related death (RR = 1.16, 95% CI 0.96–1.41; I2 = 0%, fixed effects model Fig. 4b). Regarding local recurrence, we also found that extended therapy did not significantly improve the local recurrence (RR = 0.82; 95% CI 0.64–1.06; I2 = 42 %, fixed effects model Fig. 4c), while the distant recurrence and contralateral breast cancer were increased with extended endocrine therapy, and the RR of distant recurrence and contralateral breast cancer was 0.75 (95% CI 0.58–0.96; I2 = 58%, random effects model Fig. 4d) and 0.53 (95% CI 0.40–0.70; I2 = 0%, fixed effects model Fig. 4e).
Subgroup analysis
Several subgroups analyses were performed, however, with only fewer number of studies reporting effects of size in the subgroups. Table 2 shows that the benefits of extended AIs therapy for postmenopausal women with breast cancer in reducing recurrence were associated with tumors that are > 2 cm (HR = 0.74, RD = − 0.31, P = 0.05 vs. HR = 0.85, RD = − 0.16, P = 0.20), node positive status (HR = 0.77, RD = − 0.27, P = < 0.0001 vs. HR = 0.89, RD = − 0.12, P = 0.19) and previous chemotherapy use (HR = 0.75, RD = − 0.29, P = 0.003 vs. HR = 0.91, RD = − 0.10, P = 0.44). However, the benefits of extended AIs therapy were statistically significant regardless of whether the initial endocrine therapy includes tamoxifen (HR = 0.82, RD = − 0.20, P = 0.01 vs. HR = 0.81, RD = − 0.21, P = 0.0002). Furthermore, the reduction in the risk of recurrence achieved with extended AIs therapy for postmenopausal women with breast cancer was demonstrated regardless of whether previous AIs had been used for 5 years (HR = 0.81, RD = − 0.22, P = 0.02 vs. HR = 0.78, RD = − 0.25, P = 0.01).
Toxicity
7 out of 8 trials reported toxicity and adverse events. Table 3 summarizes the common adverse events reported in the included trials. Our meta-analysis showed as was expected that extension was associated with an increase of risk of bone pain (RR = 1.26, RD = 0.04, P = 0.003, I2 = 12%), bone fractures (RR = 1.59, RD = 0.02, P = 0.002, I2 = 56 %), osteoporosis (RR = 1.53, RD = 0.07, P = 0.005, I2 = 91%), and myalgia (RR = 1.26, RD = 0.04, P = 0.02, I2 = 57%). In contrast, we did not observe a statistically significant increase in the RR of cardiovascular events (RR = 1.07, RD = 0, P = 0.41, I2 = 12%) and hypertension (RR = 0.99, RD = 0, P = 0.92, I2 = 32%) with longer durations of AIs. It was evident that bone pain, arthralgia, myalgia, osteoporosis, and fracture were higher in the extended AIs therapy group, while the cardiovascular adverse events did not increase significantly. Discontinuation of treatment for adverse events was reported in five studies [14, 16, 17, 19, 20]. Pooled data showed that longer duration of therapy with AIs was associated with a 51% increased RR for therapy discontinuation for adverse events compared with placebo or no treatment (RR = 1.51, RD = 0.06, P = 0.0009, I2 = 72%) (Table 3).
Risk of bias in included RCT studies
Full details about the risk of bias in RCT studies were shown in Fig. 5. For allocation concealment, the risk of bias was unclear in 3 RCTs with an allocation scheme that was not mentioned in the trials; in 1 study the risk of bias was low, whereas in the other 5 studies the risk of bias was high. For the performance bias, the risk of bias was unclear in 3 RCT studies and high in another one. For the detection bias, the risk was unclear in 1 study.
Due to the small number of trials that were included (< 10), no publication bias or sensitivity analysis was performed.
Discussion
The study shows that hormone receptor-positive early breast cancer recurrences continued to occur steadily throughout the study period from 5 to 20 years after 5 years of adjuvant endocrine therapy. Due to good therapeutic result and long-term toxicity of endocrine therapy of AIs, extending endocrine therapy is not routinely recommended for all patients [21, 22]. Multiple studies have assessed the hypothesis that extending the duration of endocrine therapy will result in reduction of breast cancer recurrences. Data are currently available from studies which included treatment initially with AIs-containing therapy for postmenopausal women with early breast cancer and randomized them either to ongoing AIs or placebo. Results of these studies have been mixed leading to an uncertainty regarding the benefit of extended adjuvant endocrine therapy.
The debates concerning extended adjuvant AIs therapy is obvious. The trials such as MA.17R, AERAS, and ABCSG 6a have shown a significant advantage deriving from extended endocrine therapy with AIs beyond initial AIs-containing therapy, particularly in the group of patients with higher risk of recurrence [23]. However, such improvement has not been confirmed in more recent studies. For example, NSABP B42, DATA, LATER, ABCSG 16, and IDEAL trials all failed to show an improvement in DFS and OS. Here, we report on a published data meta-analysis of eight randomized trials, showing that extended adjuvant therapy using AIs following initial AIs-containing therapy (tamoxifen, AIs or switching) in postmenopausal women provided better outcomes in terms of recurrence rate. In particular, DFS, distant recurrence and contralateral breast cancer with extended therapy were significantly better in early hormone-positive breast cancer. However, those benefits did not translate to survival benefit. Above all, the recurrence of contralateral breast cancer was significantly decreased in patients treated with extended AIs therapy. It could be argued that the extended adjuvant endocrine therapy with AIs after initial adjuvant AIs-containing therapy was a secondary prevention rather than the actual adjuvant therapy, preventing relapse of the earlier breast cancer. This preventive effect has already been shown in multiple clinical trials in healthy women without breast cancer using AIs [24,25,26,27].
In our meta-analysis, those events which were associated with substantial morbidity and mortality or commonly reported in trials would be chosen. The addition of AIs after initial adjuvant AIs-containing therapy is generally characterized by an increase in the adverse effects typical of this class of drugs, such as osteoporosis, bone pain, bone fractures, hypertension, cardiovascular events, arthralgias, myalgias and so on. The adverse events of endocrine therapy might increase due to the extended therapy. As expected, extended AIs therapy was associated with an increased RR of death without recurrence in our meta-analysis, although this was not statistically significant (1.16, P = 0.13).
Our exploratory subgroup analyses showed that tumor size (≥ T2), node positive status, previous chemotherapy use, and histological grade 1 and 2 status were independent predictors of DFS. Due to the inclusion of data from the ABCSG6a trial, histological grade 1 and 2 became an independent risk factor. However, we cannot draw firm conclusions according to the data of ABCSG Trial 6a. Because in this trial, aminoglutethimide which was used as an initial adjuvant AIs-containing therapy was no longer commonly used in the adjuvant setting now. Our results also showed that, although initial adjuvant AIs therapy was adequate for 5 years, extended use of AIs could get more benefits of DFS. Meanwhile, the potential therapeutic toxicity indicates that extended endocrine therapy was not a feasible option for all patients. A balance between toxicities and benefits must be carefully performed in cases of extended duration of therapy. Gene-expression profiles might help to identify patients who were at sufficient risk of developing late recurrences to increase efficacy and compliance [28,29,30,31]. Patients with high-risk characteristics might benefit from extended therapy.
However,it is unclear why there is a lack of extended therapy effect of survival benefit. One reason might be that events not correlated with disease recurrence could overtake the ones related to breast cancer morbidity and mortality, thus masking the actual experimental treatment benefit. Nevertheless, our results showed that extended use of AIs was not correlated with a numerical excess of deaths without breast cancer recurrence (RR = 1.16, P = 0.13). The other reason might be that, as we all know, endocrine therapy has a carryover effect with an absolute survival benefit which increases over time and becomes extremely significant in the second decade after diagnosis, when compared to the first 5 years of follow-up [4, 9]. It becomes therefore clear that an adequate follow-up period is paramount in the attempt to evaluate the benefit of extended adjuvant endocrine treatment. In this context, the carryover effect could have been lost as the follow-up period is too short.
Our work has some important limitations. First, this is a literature-based rather than an individual patient–based meta-analysis. Thus, proper subgroup analysis, including adjusting for baseline factors such as nodal and tumor size, previous chemotherapy use, the duration of initial adjuvant AIs therapy or other pathological features are not possible with the information available. Second, follow-up times are different among the trials, and in particular they are shorter for AIs. This limitation may not have captured some late deaths or relapses typically observed in this disease. Third, the heterogeneity of the outcome measures definitions across the trials, which could impact the final results. However, even in the presence of high heterogeneity between trials design characteristics, endocrine therapy duration and type, as well as differences in the outcome measures definition, our data suggest a general benefit of extended adjuvant therapy with AIs, in particular, in the patients with the high recurrence risk.
Conclusion
After initial AIs-containing adjuvant therapy, extended AIs therapy could further bring a DFS benefit for postmenopausal patients with early breast cancer, especially in the patients with high-risk characteristics. And no matter how long the duration of initial AIs-containing adjuvant therapy and whether the initial endocrine therapy included tamoxifen, extended use of AIs would lead to a further reduction in DFS. Although extended AIs therapy might increase the therapeutic toxicity and discontinuation of therapy, there were no statistically significant excess of deaths without breast cancer recurrence among patients receiving longer durations of AIs.
Availability of data and materials
All data and materials used in this research are freely available in PubMed, Embase, and Cochrane Library. References have been provided.
Abbreviations
- RCTs:
-
Randomized controlled trials
- ASCO:
-
American Society of Clinical Oncology
- SABCS:
-
San Antonio Breast Cancer
- RR:
-
Risk ratio
- HR:
-
Hazard ratio
- RD:
-
Risk difference
- CI:
-
Confidence intervals
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- N±:
-
Node positive or negative
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- LR:
-
Local recurrence
- DR:
-
Distant recurrence
- CBC:
-
Contralateral breast cancer
- NBCD:
-
Non-breast cancer-related death
- TAM:
-
Tamoxifen
- ANA:
-
Anastozole
- LET:
-
Letrozole
- AIs:
-
Aromatase inhibitors
- OFS:
-
Ovarian function suppression
- AG:
-
Aminoglutethimide
- COMB:
-
Combination
- mo:
-
Month
- plac:
-
Placebo
Reference
Rossi L, Pagani O (2015) The modern landscape of endocrine therapy for premenopausal women with breast cancer. Breast Care 10(5):312–315
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
The Early Breast Cancer Trialists’ Collaborative Group (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient level meta-analysis of randomised trials. Lancet 378:771–784
The Early Breast Cancer Trialists’ Collaborative Group (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomized trials. Lancet 386:1341–1352
Colleoni M, Sun Z, Price KN et al (2016) Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group Trials I to V. J Clin Oncol 34(9):927–935
Goss PE, Ingle JN, Martino S et al (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349:1793–1802
Mamounas EP, Jeong JH, Wickerham DL et al (2008) Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol 26:1965–1971
Davies C, Pan H, Godwin J et al (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381:805–816
Gray RG, Rea D, Handley K et al (2013) aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 31(Supplement 1):18
Kaufmann M, Jonat W, Hilfrich J et al (2007) Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 Study. J Clin Oncol 25:2664–2670
Goldvaser H, AlGorashi I, Ribnikar D et al (2017) Efficacy of extended adjuvant therapy with aromatase inhibitors in early breast cancer among common clinicopathologically defined subgroups: a systematic review and meta-analysis. Cancer Treat Rev 60:53–59
Higgins MJ, Liedke PE, Goss PE (2013) Extended adjuvant endocrine therapy in hormone dependent breast cancer: the paradigm of the NCIC-CTG MA.17/BIG 1-97 trial. Crit Rev Oncol Hematol 86:23–32
Gnant M, Steger G, Greil R et al (2018) A prospective randomized multi-center phase-III trial of additional 2 versus additional 5 years of anastrozole after initial 5 years of adjuvant endocrine therapy—results from 3,484 postmenopausal women in the ABCSG-16 trial. Cancer Res 78(4 Supplement):1
Jakesz R, Greil R, Gnant M et al (2007) Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst 99(24):1845–1853
Ohtani S, Lijima K, Higaki K et al (2019) A prospective randomized multi-center open-label phase III trial of extending aromatase-inhibitor adjuvant therapy to 10 years-Results from 1697 postmenopausal women in the N-SAS BC 05 trial: Arimidex extended adjuvant randomized study (AERAS). Cancer Res 79(4 Supplement):1
Tjan-Heijnen VCG, van Hellemond IEG, Peer PGM et al (2017) Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol 18(11):1502–1511
Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E et al (2018) Optimal duration of extended adjuvant endocrine therapy for early breast cancer; results of the IDEAL Trial (BOOG 2006-05). J Natl Cancer Inst 110(1):40–48
Zdenkowski N, Forbes JF, Boyle FM et al (2016) Observation versus late reintroduction of letrozole as adjuvant endocrine therapy for hormone receptorpositive breast cancer (ANZ0501 LATER): an open-label randomised, controlled trial. Ann Oncol 27(5):806–812
Goss PE, Ingle JN, Pritchard KI et al (2016) Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 375(3):209–219
Mamounas EP, Bandos H, Lembersky BC et al (2019) Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20(1):88–99
Burstein HJ, Prestrud AA, Seidenfeld J et al (2010) American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol 28:3784–3796
Goldhirsch A, Winer EP, Coates AS et al (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol 24:2206–2223
Goss PE, Ingle JN, Martino S et al (2005) Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 97:1262–1271
Cuzick J, Sestak I, Forbes JF et al (1922) Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet 383(9922):1041–1048
Goss PE, Ingle JN, Ales-Martinez JE et al (2011) Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 364(25):2381–2391
Howell A, Anderson AS, Clarke RB et al (2014) Risk determination and prevention of breast cancer. Breast Cancer Res 16(5):1–19
Rahman RL, Pruthi S (2012) Chemoprevention of breast cancer: the paradox of evidence versus advocacy inaction. Cancers 4(4):1146–1160
Dubsky P, Brase JC, Jakesz R et al (2013) The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer 109:2959–2964
Sgroi DC, Sestak I, Cuzick J et al (2013) Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 14:1067–1076
Gnant M, Filipits M, Greil R et al (2014) Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 risk of recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol 25:339–345
Dowsett M, Sestak I, Lopez-Knowles E et al (2013) Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 31:2783–2790
Acknowledgements
We thank Luping chen for her support and guidance throughout the project.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
W Ding designed the study and developed the analysis plan. W Ding and X.Y. Qian analyzed the data and performed meta-analysis. Z.A. Li and X.Y. Qian assessed the risk of bias. X.Y. Qian contributed in writing of the article. C.J. Tu and G.D. Ruanuan revised the manuscript and polished the language.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. Ethical approval was not applicable for this systematic review and meta-analysis.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qian, X., Li, Z., Ruan, G. et al. Efficacy and toxicity of extended aromatase inhibitors after adjuvant aromatase inhibitors-containing therapy for hormone-receptor-positive breast cancer: a literature-based meta-analysis of randomized trials. Breast Cancer Res Treat 179, 275–285 (2020). https://doi.org/10.1007/s10549-019-05464-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05464-w