Abstract
Purpose
We report the results of a retrospective analysis of the fulvestrant and palbociclib combination within a temporary authorization of use (TAU) program in 77 heavily pretreated patients with hormone receptor-positive (HR+), HER2-negative metastatic breast cancer.
Methods
All patients who received the fulvestrant and palbociclib combination within this TAU program were included. Toxicities were graded using the CTCAE v5 scale.
Results
The majority of patients (62.3%) were previously treated with the mTOR inhibitor everolimus. The median number of previous treatments for their metastatic disease was 4. With a median follow-up of 14 months, the median progression-free survival (PFS) was 7.6 months. The median PFS significantly (p < 0.0001) decreased with the number of previous treatment lines in the metastatic setting. The median PFS was 5.5 months in patients who had previously progressed on everolimus compared to 9.3 months in the everolimus non-pretreated subgroup. No significant difference in median PFS was detected in patients according to age. The median overall survival rate was not reached. The clinical benefit rate was 64%, including 4% of complete responses, 26% partial responses, and 34% stable diseases for the entire cohort.
Conclusions
The fulvestrant and palbociclib combination exerts an appreciable effect on metastatic heavily pretreated patients with a tolerable toxicity profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysregulation of the cyclin-dependent kinases (CDK) 4/6–retinoblastoma (Rb) protein pathway has been shown to contribute to the development of endocrine resistance in breast cancer [1]. The targeting of this pathway has thus been considered of interest in recent years, and several CDK inhibitors have demonstrated efficacy for the treatment of patients with hormonal receptor (HR)-positive and HER2-negative breast cancer. Palbociclib has been the first approved CDK4/6 inhibitor in combination with either letrozole in the first-line setting [2] or fulvestrant after one line of endocrine therapy [3]. The PALOMA 3 trial supported this approval, with randomly assigned patients who had relapsed or progressed during prior endocrine therapy, to receive either palbociclib and fulvestrant or placebo and fulvestrant. The results favored the palbociclib arm in terms of progression-free survival (PFS) (median PFS 9.2 months versus 5.2 months; hazard ratio 0.42; 95% confidence interval (CI): 0.32–0.56; p < 0.001) [3].
This single-center retrospective study reports the activity of palbociclib in combination with fulvestrant administered through a compassionate use program in France, known as temporary authorization of use (TAU). The TAU procedure was primarily implemented to improve early access to drugs under development or already authorized abroad, prior to its marketing authorization in Europe. In France, the present TAU was granted to palbociclib in November 2015 and restricted to postmenopausal patients with HR-positive HER2-negative metastatic breast cancer (MBC), previously exposed to multiple treatment lines, including mTOR inhibitors. This analysis assessed the efficacy and safety of palbociclib combined with faslodex in this heavily pretreated patient population.
Materials and methods
Between November 2015 and January 2017, the TAU of palbociclib was activated, though restricted to menopausal patients with HR-positive, HER2-negative MBC, previously treated with multiple treatment lines, including the combination of exemestane and everolimus. Patients received palbociclib 125 mg once a day for 3 weeks followed by 1 week off. Dose reduction to 100 mg (then 75 mg) was applied in case of Grade 4 (or febrile grade 3) neutropenia or any Grade ≥ 3 non-hematologic toxicities. Fulvestrant was given by intra-muscular injections, with a recommended dose of 500 mg once a month, and an additional 500 mg dose for 2 weeks after the first dose. Clinical outcomes and adverse events (AEs) were recorded monthly, and treatment efficacy was evaluated every three cycles. Toxicities were graded using the CTCAE v5 scale.
We herein report toxicities of the fulvestrant and palbociclib combination, as well as PFS, overall survival (OS), clinical response, and the influence of previous treatment on efficacy.
Statistical analysis
All patients alive were followed up at least until 24 May 2018. PFS was defined as the time elapsed between the date of first administration of palbociclib and the date of progression, death or 24 May 2018 (date of analysis), whichever came first. Patients alive and free of any progression at the last follow-up were censored. OS was defined as the time between the date of first administration of palbociclib and the date of death from any cause or 24 May 2018, whichever came first. Both indicators were analyzed with the Kaplan–Meier method. The logrank test was used to compare groups. Median follow-up was estimated using the reverse Kaplan–Meier method [4]. Statistical analyses were carried out by means of the SAS 9.4 statistical software (SAS Institute, Cary, NC, USA).
Results
Overall, 77 patients (75 menopaused women and 2 men), with a median age of 66 years, were treated using the TAU procedure. Patient characteristics are provided in Table 1. The median number of prior treatment lines was 4, with a median number of two lines of endocrine therapy and two lines of chemotherapy. Bone was the only metastatic site in 37.7% of patients (29/77), whereas 22.1% of patients (17/77) had exclusive visceral metastases. Only two exhibited central nervous system involvement, with one case of cerebral metastasis and one case of carcinomatous meningitis.
The median follow-up was 14 months (range 12.5–15.5). Five patients stopped treatment before the first evaluation. Treatment interruption was secondary to disease progression in 48 patients (62%) and related to toxicity for 10 patients (13%). At the date of analysis, 19 patients (25%) were still under treatment. The median duration of treatment was 122 days, with the best response evaluable in 72 patients. Three patients (4%) had a complete response, 20 patients (26%) partial response, 26 patients (34%) stable disease (for at least 24 weeks), and 23 patients (30%) had progressive disease.
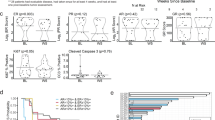
The median PFS was 7.6 (range 4.6–10.4) months for the entire cohort (Fig. 1), with the median OS not reached. Overall, 48 (62.3%) patients were pretreated with exemestane and everolimus combination. Of these 48, 22 patients stopped everolimus because of progression, whereas 26 interrupted the therapy due to toxicity. The median PFS in the everolimus non-pretreated subgroup was 9.3 (95% CI [6.0–12.3]) months, as compared to 5.5 (95% CI [3.0–11.0]) months in patients who had previously progressed under everolimus (Fig. 2). This difference was not statistically significant (p = 0.19). The median PFS significantly (p < 0.0001) decreased with the number of previous treatment lines in a metastatic setting (Fig. 3). The median PFS was 10.5 months with ≤ 2 previous lines of treatment, 8.8 months with three to four previous lines, 7.6 months with five to six previous lines, 8.7 months with seven to eight previous lines, and 2.9 months with more than eight previous treatment lines. No significant difference in median PFS was detected in patients according to age.
The most common grade 3/4 AEs were neutropenia in 48 patients (63%), with only one patient exhibiting febrile neutropenia, asthenia in 8 patients (10.4%), thrombopenia in 5 patients (6.5%) and anemia in 2 patients (2.6%). Because of toxicity, 24 patients (31%) had a dose reduction to 100 mg, and four patients (5%) to 75 mg. Table 2 summarizes the toxicity profile in our cohort.
Discussion
Palbociclib is the first-in-class CDK4/6 inhibitor approved for HR-positive MBC. Pivotal registration trial PALOMA-3 assessed its efficacy in combination with fulvestrant in patients whose disease progressed during prior ET [3]. This trial met its primary endpoint with an improvement in PFS for the fulvestrant plus palbociclib arm versus fulvestrant plus placebo arm (9.5 versus 4.6 months, p < 0.0001, respectively). Secondary endpoints favored the combination arm with a higher response rate (25.0 versus 11.1%, p = 0.0012) than the control arm. Recently, an updated analysis showed a nonstatistically significant improvement in OS in the entire population (34.9 versus 28.0 months in the experimental arm versus the control arm, p = 0.09) [5].
Beyond first- and second-line, questions arise concerning the activity of CDK 4/6 inhibitors. The Phase II TREnd trial randomized 115 postmenopausal patients diagnosed with HR-positive, HER2-negative MBC to receive palbociclib either alone or in combination with their current endocrine therapy (aromatase inhibitor or fulvestrant) [6]. These patients had progressed after one or two endocrine treatments. The median PFS was significantly longer with the combination of endocrine therapy and palbociclib (median PFS, 11.5 vs. 6 months for palbociclib alone; HR = 0.35 [0.18–0.7]; p = 0.002). The authors concluded that palbociclib was likely to reverse the acquired resistance to the identical endocrine agent used in the previous endocrine therapy lines.
Four retrospective studies evaluated the activity of the fulvestrant–palbociclib combination in heavily pretreated patients (Table 3). The Roswell Park Comprehensive Cancer Center reported data on 23 everolimus-pretreated patients [7]. There was no response, with a PFS of 2.9 months. The Jules Bordet Institut experience was reported with 34 patients [8]. The median PFS was 3.1 months with no difference between mTOR inhibitor-pretreated (3.5 months) and inhibitor-naïve patients (2.7 months; HR = 0.83). Data from 60 patients were analyzed in the René Gauducheau Cancer Center [9]. All patients were pretreated using everolimus, and the median PFS was 5.8 months. Lastly, the UK compassionate access program experience was published, with 118 patients included [10]. Therein, palbociclib was associated with various endocrine treatments. After a median number of five previous treatments, the median PFS was 4.5 months, with a median OS of 15.8 months.
In the present study, PFS was longer for patients without previous everolimus exposure compared to those with previous progression on everolimus (9.3 versus 5.5 months). Owing to the small number of patients, this difference was not statistically significant (p = 0.19). Nevertheless, this result is different from what was observed in the Belgian study [8].
The efficacy of the fulvestrant + palbociclib combination was strongly related to the number of previous treatments, with a significant decrease of median PFS (p < 0.0001) with the number of previous treatment lines in the metastatic setting (Fig. 3). In the subset of patients with one to two previous treatment lines (19 patients), the median PFS was 10.5 months. This result is consistent with the median PFS of 9.9 months in patients treated with the fulvestrant and palbociclib combination in the PALOMA 3 trial, with a median number of previous metastatic treatments of one in a metastatic setting [3].
The haematologic safety profile of palbociclib in our study was very similar to that of PALOMA-3 trial [3]. Grade 3–4 neutropenia was reported in 62.4% of patients in our series and in 62% of PALOMA 3 patients, grade 3–4 thrombopenia in 6.5% of patients in our series and in 2.3% of PALOMA 3 patients, grade 3–4 anemia in 2.6% of patients in our series and in PALOMA 3 patients.
In conclusion, this single-center retrospective study revealed a median PFS of 7.6 months, with a favorable toxicity profile, in 77 heavily pretreated patients. The median PFS was significantly better for patients with few previous treatment lines in metastatic setting and seemed better without previous everolimus treatment. The median PFS was not influenced by age.
References
Finn RS, Aleshin A, Slamon DJ (2016) Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res 18(1):17–28. https://doi.org/10.1186/s13058-015-0661-5
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Diéras V, Slamon DJ (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375(20):1925–1936. https://doi.org/10.1056/NEJMoa1607303
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Zhang K, Theall KP, Jiang Y, Bartlett CH, Koehler M, Slamon D (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17(4):425–439. https://doi.org/10.1016/S1470-2045(15)00613-0
Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17(4):343–346
Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, André F, Puyana Theall K, Huang X, Giorgetti C, Huang Bartlett C, Cristofanilli M (2018) Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 379(20):1926–1936. https://doi.org/10.1056/NEJMoa1810527
Malorni L, Curigliano G, Minisini AM, Cinieri S, Tondini CA, D’Hollander K, Arpino G, Bernardo A, Martignetti A, Criscitiello C, Puglisi F, Pestrin M, Sanna G, Moretti E, Risi E, Biagioni C, McCartney A, Boni L, Buyse M, Migliaccio I, Biganzoli L, Di Leo A (2018) Palbociclib as single agent or in combination with the endocrine therapy received before disease progression for estrogen receptor-positive, HER2-negative metastatic breast cancer: TREnd trial. Ann Oncol 29(8):1748–1754. https://doi.org/10.1093/annonc/mdy214
Dhakal A, Matthews CM, Levine EG, Salerno KE, Zhang F, Takabe K, Early AP, Edge SB, O’Connor T, Khoury T, Young JS, Opyrchal M (2018) Efficacy of palbociclib combinations in hormone receptor-positive metastatic breast cancer patients after prior everolimus treatment. Clin Breast Cancer 18(6):1401–1405. https://doi.org/10.1016/j.clbc.2018.04.015
Maurer C, Ferreira AR, Martel S, Lambertini M, Pondé N, Aftimos P, de Azambuja E, Piccart M (2018) Endocrine therapy and palbociclib within a compassionate use program in heavily pretreated hormone receptor-positive, HER2-negative metastatic breast cancer. Breast 39:14–18. https://doi.org/10.1016/j.breast.2018.02.027
du Rusquec P, Palpacuer C, Campion L, Patsouris A, Augereau P, Gourmelon C, Robert M, Dumas L, Caroline F, Campone M, Frenel JS (2018) Efficacy of palbociclib plus fulvestrant after everolimus in hormone receptor-positive metastatic breast cancer. Breast Cancer Res Treat 168(2):559–566. https://doi.org/10.1007/s10549-017-4623-8
Battisti NML, Kingston B, King J, Denton A, Waters S, Sita-Lumsden A, Rehman F, Stavraka C, Kristeleit H, Sawyer E, Houghton D, Davidson N, Howell S, Choy J, Harper P, Roylance R, Fharat R, Mohammed K, Ring A, Johnston S (2019) Palbociclib and endocrine therapy in heavily pretreated hormone receptor-positive HER2-negative advanced breast cancer: the UK Compassionate Access Programme experience. Breast Cancer Res Treat 174(3):731–740. https://doi.org/10.1007/s10549-019-05134-x
Author information
Authors and Affiliations
Contributions
MV and TP: conceived the study. HH and JL: collected and categorized the clinical data. MV, TP and XP: performed the statistical analyses and interpreted the results. HH: drafted the manuscript and all authors critically revised and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Thierry Petit is member of a Pfizer scientific board.
Ethical approval
The study was approved by the Local Ethical Review Board, and complies with the current laws of France. The study has been performed in accordance with the 1964 Helsinki Declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Herrscher, H., Velten, M., Leblanc, J. et al. Fulvestrant and palbociclib combination in heavily pretreated hormone receptor-positive, HER2-negative metastatic breast cancer patients. Breast Cancer Res Treat 179, 371–376 (2020). https://doi.org/10.1007/s10549-019-05439-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05439-x