Abstract
Purpose
Aromatase inhibitor-associated musculoskeletal symptoms (AIMSS) are common adverse events of AIs often leading to drug discontinuation. We initiated a prospective clinical trial to evaluate whether bisphosphonates are associated with reduced incidence of AIMSS.
Methods
In the single-arm trial, the Zoledronic Acid Prophylaxis (ZAP) trial, we compared the incidence of AIMSS against historical controls from the Exemestane and Letrozole Pharmacogenomics (ELPh) trial. Eligible women were postmenopausal with stage 0-III breast cancer planning to receive adjuvant AIs. AIMSS was assessed using the Health Assessment Questionnaire and Visual Analog Scale over 12 months in both trials. Participants in the ZAP trial received zoledronic acid prior to initiating letrozole and after 6 months; ELPh participants included in the analysis were taking letrozole but not bisphosphonates. We analyzed patient-reported outcomes (PROs) and bone density in the ZAP trial using mixed-effects linear regression models and paired t tests, respectively.
Results
From 2011 to 2013, 59 postmenopausal women enrolled in ZAP trial. All 59 (100%) women received baseline and 52 (88%) received 6-month zoledronic acid, and had similar characteristics to historical controls from the ELPh trial (n = 206). Cumulatively during the first year of AI, 37 and 67% of ZAP and ELPh participants reported AIMSS (p < 0.001), respectively. Within the ZAP trial, we did not observe significant changes in other PROs; however, we report improvements in bone mineral density.
Conclusions
Compared to historical controls, zoledronic acid administered concomitantly with adjuvant AIs was associated with a reduced incidence of AIMSS. A randomized controlled trial is required to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adjuvant aromatase inhibitors (AI) reduce the risk of breast cancer recurrence and death in postmenopausal women with early-stage hormone receptor-positive disease [1]. While all three approved third generation AIs (exemestane, anastrozole, letrozole) reduce the concentration of circulating estrogens by inhibiting aromatase and improve breast cancer outcomes, these agents are associated with common toxicities leading to discontinuation of therapy [2]. Studies have demonstrated that 25–32% of women will discontinue AI prior to completing a 5-year course, and in studies where 10 years of AI therapy was recommended, adherence decreased to 38% [2,3,4]. A common cause for AI discontinuation is AI-associated musculoskeletal symptoms (AIMSS), which result in an 18–24% discontinuation rates during the first 2 years [2, 5].
Up to 61% of women treated with AIs experience AIMSS, which adversely affects quality of life [2, 6]. Factors that may contribute to AIMSS include prior chemotherapy, in particular taxanes, obesity, and the interval time since menopause [7]. Previous studies have suggested that exercise, non-steroidal anti-inflammatory drugs, acetaminophen, opioids, dietary supplementation, vitamin D, serotonin and norepinephrine reuptake inhibitors, and acupuncture may improve AIMSS; however, no confirmatory studies have been reported and there are currently no formal guidelines [7,8,9,10,11].
In women with skeletal metastasis from breast cancer bisphosphonates can decrease bone-related pain and improve quality of life [12, 13]. Furthermore, a retrospective study of women treated with AIs has demonstrated that women taking bisphosphonates and calcium were less likely to report arthralgias [14]. Bisphosphonates are a particularly attractive class of agents for management of AIMSS because they are well-tolerated, have been associated with reduced bone loss and improved bone mineral density (BMD) in patients treated with AIs, and may also be associated with improved clinical outcomes in states of estrogen-depletion [15, 16]. We hypothesized that women prescribed zoledronic acid while taking adjuvant AI would be less likely to report AIMSS compared to historical controls in a similar population of women taking adjuvant AIs but not taking bisphosphonates.
Materials and methods
Study design
We conducted a single-arm prospective clinical trial in postmenopausal women with ductal carcinoma in situ (DCIS) or stage I-III breast cancer scheduled to initiate adjuvant AI who were not taking bisphosphonates, designated the Zoledronic Acid Prophylaxis (ZAP) trial. Eligible women received 4 mg of intravenous zoledronic acid at baseline and 6 months. Participants started 2.5 mg of daily oral letrozole 1–2 weeks following the initial dose of zoledronic acid. As directed per protocol, the percentage of women who developed AIMSS over 12 months was compared to historical controls from the Exemestane and Letrozole Pharmacogenetics (ELPh) trial (NCT00263913) who were not taking bisphosphonates. The ELPh trial was a prospective multicenter randomized observational open-label trial in postmenopausal women with early-stage breast cancer (stage 0-III) evaluating the effects of 2 years of therapy with either letrozole or exemestane on a variety of biomarkers of estrogen activity and potential AI toxicity, including a prospective evaluation of AIMSS; these results have been previously published [8, 17]. Participants on the ELPh trial who were randomized to letrozole not taking bisphosphonates at any point during the initial 12 months were included in this analyses as historical controls; those randomized to exemestane or taking bisphosphonates were excluded.
The primary objective of this analysis was to compare the percentage of participants reporting AIMSS taking letrozole in both the ZAP and ELPh trials. Secondary objectives examined differences in the Health Assessment Questionnaire Disability Index (HAQ-DI) and pain scores on the Visual Analog Scale (VAS), and AI discontinuation rates between participants of the two studies [2, 8, 9]. We assessed additional patient-reported outcomes (PROs) evaluating depression, anxiety, hot flashes, menopausal symptoms, sleep quality, and overall quality of life, and determined changes in BMD, over 12 months.
Assessment of AIMSS
AIMSS in both studies was defined using the HAQ-DI and VAS instruments, using methods which have been previously validated [2, 8, 9]. AIMSS were defined as an increase of 0.22 in a scale of 0–3 in the HAQ-DI and/or an increase of 2.0 cm in a scale of 10 cm in the VAS. In both studies we assessed AIMSS using the HAQ-DI and VAS questionnaires at 1, 3, 6, and 12 months.
Collection of other patient-reported outcomes
Additional PROs were collected using validated questionnaires at baseline, 1, 3, 6, and 12 months, and included measures of depression, anxiety, hot flashes, menopausal symptoms, sleep quality, and overall quality of life. Depression or depressive symptoms were assessed via the Center for Epidemiologic Studies Depression Scale (CESD), where responses are summed to obtain total scores ranging from 0 to 60, and a higher score reflects more depressive symptoms [18]. Anxiety was assessed with the anxiety subscale of the Hospital Anxiety and Depression Scale (HADS-A); total HADS-A score was computed by summing scores ranging from 0 to 21, where a higher score reflects more depressive and anxiety symptoms [19]. The Hot Flash Related Daily Interference Scale (HFRDI) was used to assess the severity of hot flashes. Participants rated the degree to which hot flashes had interfered with each item during the previous week using a 0 (do not interfere) to 10 (completely interfere) scale, and a total score was computed by summing items and ranged from 0 to 100 [20]. Menopausal symptoms were assessed using the revised National Surgical Adjuvant Breast and Bowel Project (NSABP) menopausal symptoms questionnaire assessing 47 common symptoms. A total score was computed by summing items and ranged from 0 to 90 where higher scores reflect more menopausal symptoms [21]. Sleep quality and disturbance were assessed using the well-validated Pittsburgh Sleep Quality Index (PSQI), where a global PSQI score was computed by summing scores from the seven component scores and ranged from 0 to 21 and scores 5 or greater suggest poor sleep quality [22]. An overall rating of quality of life was assessed using the EuroQol. Participants were asked to provide a single number to describe their own health state that day using a Cantril-like ladder scale with best health at the top (100) and worst health (death) at the bottom (0) [23]. Participants were also asked to provide a rating for an average woman their own age for use as a comparison standard to judge their own rating.
Bone mineral density
BMD was measured in the anterior-posterior spine, femoral neck, trochanter, and total hip at baseline and after 12 months using dual-energy X-ray absorptiometry (DEXA) scans. T scores were extracted from individual scan reports, and T scores in their respective bones assessed at baseline and 1 year.
Statistical approach
Sample size was calculated based on the assumption that zoledronic acid would reduce the frequency of AIMSS after 12 months of treatment from the expected frequency of 50–30% within the ZAP trial. Allowing for a 20% drop out rate of the 59 planned patients for accrual, 47 evaluable patients for analysis would yield 80% power to detect an improvement in the frequency of AIMSS from 50 to 30% with a two-sided type 1 error of 5%. Characteristics of participants and scores at baseline were summarized with descriptive measures and compared between studies with Fisher’s exact test and Wilcoxon rank sum tests. AIMSS was calculated based on HAQ-DI and VAS scores as described above, and were summarized at 1, 3, 6 and 12 months for each study. The development of AIMSS at any time in the first year was explored between studies using a logistic regression model adjusting for patient age, prior chemotherapy, and prior tamoxifen use where data were available. Differences between studies was explored in subgroups (age, prior therapy, BMI, baseline VAS) using interaction analyses. Differences in the frequency of AIMSS at each time point between studies were estimated longitudinally using linear contrasts and summarized with odds ratios from a multivariable mixed-effects logistic regression model that included fixed effects for study-by-time point main effects and interaction, age and prior chemotherapy as covariates, and a random intercept for each patient to account for repeated measures.
Changes from baseline in HAQ-DI and VAS between studies were estimated by dichotomizing the HAQ-DI and VAS scores as 0 versus > 0. Multivariable mixed-effects logistic regression models and linear contrasts were used to estimate odds ratios for comparing ZAP to ELPh each follow-up time point relative to baseline. One model was fit for each outcome with fixed effects for study-by-time point main effects and interaction, age, prior chemotherapy, and prior tamoxifen use, and a random intercept for each subject. The cumulative probability of AI discontinuation or change to another endocrine therapy by 1 year was evaluated using the Kaplan–Meier method.
Changes from baseline in PROs for the ZAP cohort were analyzed using mixed-effects linear regression models that included the log-scale PRO as the dependent variable and fixed effect indicator variables for the 3 follow-up time points and a random intercept for each patient to account for repeated measures. Frequency distributions of BMD at baseline and 12 months were assessed. BMD and T score data are summarized at baseline and 12 months using means and standard deviations (SD). Changes in BMD and T scores were analyzed using paired t tests.
Results
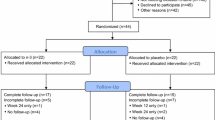
From February 2011 to January 2013, 63 women were consented for the ZAP study. Of these, three women were found to be ineligible during screening (two based on creatinine clearance < 50 mL/min, and one due to metastatic disease observed on routine imaging). Of the 60 eligible women 59 eligible are included in the analysis (Fig. 1). All 59 (100%) women who enrolled received baseline zoledronic acid and 52 (88%) received the 6-month dose. Of the 7 who did not receive the 6-month dose, four withdrew consent or came off study early for reasons not otherwise specified; one decided to switch to tamoxifen; one decided not to receive the follow-up dose due to bone and jaw pain (not diagnosed with osteonecrosis of the jaw); and one had recurrent disease, requiring study discontinuation. Of the 503 women enrolled in the ELPh study from August 2005 until July 2009, 206 were eligible as historical controls (Fig. 1). A higher proportion of women in the ELPh trial received prior tamoxifen, and had higher VAS and HAQ-DI scores at baseline; however, there were no additional differences between cohorts that were statistically significant (Tables 1, 2).
Women on the ZAP trial tolerated zoledronic acid well with no major toxicity, including no reports of osteonecrosis of the jaw or documented hypocalcaemia. The most common side effects attributed to zoledronic acid reported by women in the ZAP study were flu-like symptoms (n = 23, 39%) and gastrointestinal side effects (n = 14, 24%).
Participants in the ELPh cohort had higher VAS and HAQ-DI scores at baseline compared to the ZAP cohort. HAQ-DI scores stayed the same in ELPh and ZAP (interaction p-value = 0.23), however, VAS scores increased in ELPh but remained stable in ZAP (interaction p-value = 0.02). Over the 12-month study period, VAS and HAQ-DI scores remained higher in the ELPh cohort compared to the ZAP cohort (Table 2). When both HAQ-DI and VAS were scored to assess AIMSS, presence of AIMSS at each time point throughout the year was significantly lower in ZAP compared to the ELPh cohort (Fig. 2). Participants on ZAP were significantly less likely to report AIMSS at any point in the first 12 months (odds ratio (OR) = 0.28, 95% confidence interval (CI) 0.14–0.53, p < 0.0001, adjusted for baseline VAS score and prior use of tamoxifen). There was no interaction between study cohort and prior tamoxifen use or study cohort and baseline VAS on reporting AIMSS in the first 12 months. Exploratory subgroup analyses found that participants in ZAP were less likely to report AIMSS irrespective of age, prior taxanes or tamoxifen use, BMI, or baseline VAS scores (Supplementary Fig. 1). Five (8%) women on the ZAP study, and 22 (11%) on the ELPh study discontinued AI or changed endocrine therapy during the first year (p = 0.81). The cumulative probability of discontinuing AI or changing endocrine therapy by 1 year was 9% for ZAP and 14% for ELPh (log rank p = 0.57). None (0%) of the ZAP participants discontinued AI after the 6 month time point, compared to nine (5%) discontinuations on ELPh (Table 3).
Analysis of other PROs within the ZAP trial did not reveal any significant change at any time point during the 12-month study period compared to baseline (Fig. 3). However, by 12 months, ZAP participants rated their overall quality of life (EuroQol) higher compared to what they felt those their own age would have.
Patient reported outcomes (PRO) in ZAP participants over 12 months assessing changes in (a) depression symptoms (Center for Epidemiologic Studies Depression Scale, CESD), b anxiety and depression (Hospital Anxiety and Depression Scale, HADS-A), c hot flashes (Hot Flash Related Daily Interface Scale, HFRDI), d menopausal symptoms (National Surgical Adjuvant Breast and Bowel Project menopausal symptoms questionnaire, NSABP Menopausal Score), e sleep quality (Pittsburgh Sleep Quality Index, PSQI), and f overall quality of life
In the ZAP study, 30 patients had normal BMD (52%), 24 had osteopenia (41%), and 4 had osteoporosis (7%) at baseline. Of the 48 patients with 12 month BMD data, 42 remained with normal BMD (88%) and four patients had an improvement in BMD category (8%), while only two patients had declines in BMD category (4%). Furthermore, mean T score in the trochanter improved by 0.12 (95% CI 0–0.23, p = 0.046), and both mean T score and BMD in the spine improved by 0.23 (95% CI 0.13–0.33, p < 0.001) and 0.03 (95% CI 0.01–0.04, p < 0.001), respectively (Supplementary Table 1).
Discussion
This is the first prospective study to demonstrate that the administration of zoledronic acid in the adjuvant setting, compared to historical controls, is associated with a decreased incidence of AIMSS in women taking adjuvant letrozole. Additional exploratory analysis demonstrated probability of AI discontinuation in women using zoledronic acid was numerically lower than in those not receiving zoledronic acid (9 versus 14 month time to discontinuation (TTD), Table 3), although this was not statistically significant (HR = 0.87, 95% CI 0.32–2.35, p = 0.57). These findings are particularly important since AIMSS is a leading cause of premature AI discontinuation.
Bisphosphonates have anti-inflammatory properties, and studies have found they decrease cytokines such as tumor necrosis factor alpha, and interleukin 1 and 6 [24, 25]. Furthermore, in patients with rheumatoid arthritis, bisphosphonates have been shown to deplete synovial macrophages and decrease expression of adhesion molecules in the synovial lining [26]. Estrogens have been found to have anti- and pro-inflammatory properties, thus by decreasing estrogens, AIs may be increasing local inflammation in synovial fluid leading to pain [27, 28]. The anti-inflammatory effects of bisphosphonates, especially in synovial fluid, may explain our findings, although additional research is needed to elucidate the pathology of AIMSS and the effects of bisphosphonates.
The ZAP trial is a single-arm prospective study. Thus, results may be influenced by a placebo effect. While a placebo effect cannot be fully mitigated in a single-arm study, to compare the effects of bisphosphonates on AIMSS we used a similar cohort of women taking AIs from another prospective study with similar entry criteria as historical controls. Both the ZAP and ELPh trials were conducted close to the same time, and participants underwent evaluation of AIMSS at the same intervals using identical tools. Both cohorts had similar characteristics, with the exception of previous tamoxifen use and baseline VAS scores. Previous use of tamoxifen has been associated with joint stiffness; however, data from the ELPh trial have not demonstrated that tamoxifen use predicts AIMSS [8, 29]. ELPh participants reported higher VAS scores at baseline compared to ZAP participants; however, association of high baseline VAS scores with AI discontinuation is weak (HR 1.09, 95% CI 0.99–1.2, p = 0.067) [30]. Indeed, adjusted analysis for prior tamoxifen use and higher baseline VAS did not change our primary findings. Furthermore, exploratory subgroup analysis including age, BMI, those who previously received taxanes or tamoxifen, and baseline VAS scores, found that participants in ZAP reported improve rates of AIMSS.
While ZAP participants had a decreased probability of AI discontinuation compared to ELPh participants, this result did not reach statistical significance. These results, however, must be regarded as exploratory, since the ZAP study was not designed or powered to assess this endpoint. Rates of AI discontinuation are particularly relevant as recent data suggest extended AI regimens may improve disease free survival, and increasing data suggest that AIs may have a role in the adjuvant treatment of premenopausal women [4, 31].
While the recommended duration of adjuvant AI therapy is 5 to 10 years, in this pilot study we only evaluated AIMSS over 1 year. Although the every 6 month schedule used in this study could potentially be extended through 5 years, the majority of AIMSS are usually reported within the first 3 months and peak by 6 months [6, 8].
Although we did not observe a difference among various PROs within the ZAP trial, participants consistently reported superior perception of quality of life compared to those their own age. Similar improvements in overall quality of life have been described in patients with metastatic breast cancer taking bisphosphonates [32]. Furthermore, our study shows that 96% of patients either maintained a normal bone density or had an improvement in BMD category, and mean T scores in the trochanter and spine improved, demonstrating the known beneficial effects of bisphosphonates on bone health. Other studies have also confirmed that the benefit of zoledronic acid on BMD translates to improvements in preventing fractures [33].
Our results contribute additional rationale for the potential role of bisphosphonates in the adjuvant setting for women taking AIs. Bisphosphonates are approved for the treatment of osteoporosis by improving BMD and decreasing the risk of fractures. The detrimental effects on BMD caused by AI therapy are well studied, and we demonstrate as others have that bisphosphonates can reverse this effect [34, 35]. As studies suggest extended AI therapy may modestly improve rates of breast cancer recurrence, bone health will become an increasingly relevant factor [36]. Furthermore, several studies have demonstrated decreased rates of skeletal metastasis and improved survival in women taking adjuvant bisphosphonates for early breast cancer, particularly in estrogen-depleted states where AI therapy would be a consideration [16, 37, 38].
In summary, our data suggest that bisphosphonates are associated with reduced incidence of AIMSS in postmenopausal women with early breast cancer treated with letrozole. While these data suggest bisphosphonates may have important implications in bone health, they may also impact survival either through direct anti-cancer mechanisms or improving tolerance to AI therapy. These results add additional evidence and rationale for the potential role of bisphosphonates in the adjuvant setting; therefore, definitive randomized prospective studies evaluating the role of bisphosphonates in the adjuvant setting to prevent AIMSS are warranted.
References
Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M et al (2010) Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28(3):509–518
Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, Hayden J, Tarpinian K, Yakim E, Flockhart DA et al (2012) Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 30(9):936–942
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW (2012) Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 134(2):459–478
Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, Gelmon K, Whelan T, Strasser-Weippl K, Rubin S et al (2016) Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J Med 375(3):209–219
Lombard JM, Zdenkowski N, Wells K, Beckmore C, Reaby L, Forbes JF, Chirgwin J: Aromatase inhibitor induced musculoskeletal syndrome: a significant problem with limited treatment options. Support Care Cancer 2015
Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, Greene BT, DeMichele A (2009) Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer 115(16):3631–3639
Gaillard S, Stearns V (2011) Aromatase inhibitor-associated bone and musculoskeletal effects: new evidence defining etiology and strategies for management. Breast Cancer Res 13(2):205
Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, Hayden J, Lemler S, Shahverdi K, Powers P et al (2008) Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat 111(2):365–372
Henry NL, Banerjee M, Wicha M, Van Poznak C, Smerage JB, Schott AF, Griggs JJ, Hayes DF (2011) Pilot study of duloxetine for treatment of aromatase inhibitor-associated musculoskeletal symptoms. Cancer 117(24):5469–5475
Mao JJ, Xie SX, Farrar JT, Stricker CT, Bowman MA, Bruner D, DeMichele A (2014) A randomised trial of electro-acupuncture for arthralgia related to aromatase inhibitor use. Eur J Cancer 50(2):267–276
Irwin ML, Cartmel B, Gross CP, Ercolano E, Li F, Yao X, Fiellin M, Capozza S, Rothbard M, Zhou Y et al (2015) Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol 33(10):1104–1111
Pavlakis N, Schmidt R, Stockler M: Bisphosphonates for breast cancer. Cochrane Database Syst Rev 2005(3):CD003474
Wardley A, Davidson N, Barrett-Lee P, Hong A, Mansi J, Dodwell D, Murphy R, Mason T, Cameron D (2005) Zoledronic acid significantly improves pain scores and quality of life in breast cancer patients with bone metastases: a randomised, crossover study of community vs hospital bisphosphonate administration. Br J Cancer 92(10):1869–1876
Muslimani AA, Spiro TP, Chaudhry AA, Taylor HC, Jaiyesimi I, Daw HA (2009) Aromatase inhibitor-related musculoskeletal symptoms: is preventing osteoporosis the key to eliminating these symptoms? Clin Breast Cancer 9(1):34–38
Bundred NJ, Campbell ID, Davidson N, DeBoer RH, Eidtmann H, Monnier A, Neven P, von Minckwitz G, Miller JC, Schenk NL et al (2008) Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST Study results. Cancer 112(5):1001–1010
Early Breast Cancer Trialists’ Collaborative G, Coleman R, Powles T, Paterson A, Gnant M, Anderson S, Diel I, Gralow J, von Minckwitz G, Moebus V et al: Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 2015, 386(10001):1353–1361
Santa-Maria CA, Blackford A, Nguyen AT, Skaar TC, Philips S, Oesterreich S, Rae JM, Desta Z, Robarge J, Henry NL et al (2016) Association of variants in candidate genes with lipid profiles in women with early breast cancer on adjuvant aromatase inhibitor therapy. Clin Cancer Res 22(6):1395–1402
LS R (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1(3):385–401
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6):361–370
Carpenter JS (2001) The Hot Flash Related Daily Interference Scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manag 22(6):979–989
Stanton AL, Bernaards CA, Ganz PA (2005) The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst 97(6):448–456
Buysse DJ, Reynolds CF, Monk TH, Hoch CC, Yeager AL, Kupfer DJ (1991) Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep 14(4):331–338
Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A (1997) Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol. Br J Rheumatol 36(5):551–559
Ceponis A, Waris E, Monkkonen J, Laasonen L, Hyttinen M, Solovieva SA, Hanemaaijer R, Bitsch A, Konttinen YT (2001) Effects of low-dose, noncytotoxic, intraarticular liposomal clodronate on development of erosions and proteoglycan loss in established antigen-induced arthritis in rabbits. Arthritis Rheum 44(8):1908–1916
Cantatore FP, Acquista CA, Pipitone V (1999) Evaluation of bone turnover and osteoclastic cytokines in early rheumatoid arthritis treated with alendronate. J Rheumatol 26(11):2318–2323
Barrera P, Blom A, van Lent PL, van Bloois L, Beijnen JH, van Rooijen N, de Waal Malefijt MC, van de Putte LB, Storm G, van den Berg WB: Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum 2000, 43(9):pp 1951–1959
Capellino S, Straub RH, Cutolo M (2014) Aromatase and regulation of the estrogen-to-androgen ratio in synovial tissue inflammation: common pathway in both sexes. Ann N Y Acad Sci 1317:24–31
Schmidt M, Hartung R, Capellino S, Cutolo M, Pfeifer-Leeg A, Straub RH (2009) Estrone/17beta-estradiol conversion to, and tumor necrosis factor inhibition by, estrogen metabolites in synovial cells of patients with rheumatoid arthritis and patients with osteoarthritis. Arthritis Rheum 60(10):2913–2922
Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, Sierra A, Hershman DL (2007) Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol 25(25):3877–3883
Kidwell KM, Harte SE, Hayes DF, Storniolo AM, Carpenter J, Flockhart DA, Stearns V, Clauw DJ, Williams DA, Henry NL (2014) Patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer 120(16):2403–2411
Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Lang I, Gomez HL, Tondini C, Burstein HJ, Perez EA et al (2014) Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 371(2):107–118
Diel IJ, Body JJ, Lichinitser MR, Kreuser ED, Dornoff W, Gorbunova VA, Budde M, Bergstrom B, Group MFS (2004) Improved quality of life after long-term treatment with the bisphosphonate ibandronate in patients with metastatic bone disease due to breast cancer. Eur J Cancer 40(11):1704–1712
Coleman R, Cameron D, Dodwell D, Bell R, Wilson C, Rathbone E, Keane M, Gil M, Burkinshaw R, Grieve R et al (2014) Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol 15(9):997–1006
Lustberg MB, Reinbolt RE, Shapiro CL (2012) Bone health in adult cancer survivorship. J Clin Oncol 30(30):3665–3674
Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar R, Litsas G, McKay R, Podoloff DA, Srinivas S et al (2013) NCCN task force report: bone health in cancer care. J Natl Compr Cancer Netw 11(3):S1-50 quiz S51.
Higgins MJ, Liedke PE, Goss PE (2013) Extended adjuvant endocrine therapy in hormone dependent breast cancer: the paradigm of the NCIC-CTG MA.17/BIG 1–97 trial. Crit Rev Oncol Hematol 86(1):23–32
Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, von Minckwitz G, Sleeboom HP, Forbes J, Barrios C et al (2013) Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol 24(2):398–405
Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M, Gil M, Houston SJ, Grieve RJ, Barrett-Lee PJ et al (2011) Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med 365(15):1396–1405
Acknowledgements
Zoledronic acid and letrozole were provided by Novartis.
Funding
Breast Cancer Research Foundation, National Institutes of Health [P30 CA06973].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CAS has received research funding from Medimmune and Pfizer. VS received research funding from Abbvie, Medimmune, Novartis, Pfizer, and PUMA. CS received research funding from Genentech (to the institution); speaking honorarium from CaretMD and Tower Health System; royalties from UptoDate; and travel support from Optum. RC received research funding from Merck, Novartis, Merrimack, Clovis, Genentech, and PUMA. AW has received research funding from Pfizer. NLH received research funding from AstraZeneca. All remaining authors have declared no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.

10549_2018_4811_MOESM1_ESM.jpg
Subgroup analysis assessing AIMSS at any time point according to age, prior taxanes, prior tamoxifen, BMI, and baseline VAS scores. Supplementary material 1 (JPG 430 KB)
Supplementary material 2 (DOCX 12 KB)
Rights and permissions
About this article
Cite this article
Santa-Maria, C.A., Bardia, A., Blackford, A.L. et al. A phase II study evaluating the efficacy of zoledronic acid in prevention of aromatase inhibitor-associated musculoskeletal symptoms: the ZAP trial. Breast Cancer Res Treat 171, 121–129 (2018). https://doi.org/10.1007/s10549-018-4811-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4811-1