Abstract
Purpose
Patients treated with trastuzumab for HER2-positive metastatic breast cancer (HER2+MBC) are living longer, but there is little information on their outcomes and treatment experience beyond the median survival from clinical trials and real-world observational studies. We aim to describe the real-world treatment patterns and overall survival (OS) for women surviving five or more years from initiation of trastuzumab for HER2+MBC.
Methods
This is a retrospective, whole-of-population cohort study of women initiating trastuzumab for HER2+MBC between 2001 and 2011, followed to 2016. We defined long-term survivors (LTS) as those patients surviving ≥ 5 years from trastuzumab initiation. We used dispensing claims to describe timing of cancer treatments used by LTS and to estimate time on and off HER2-targeted therapies, and OS from trastuzumab initiation for HER2+MBC.
Results
Of 4177 women initiating trastuzumab for HER2+MBC, 1082 (26%) survived ≥ 5 years. Median age for LTS was 54 years (IQR 46–63). At a median follow-up of 9.4 years, 36% of LTS died; their conditional probability of surviving an additional 5 years was 55%. Median time on trastuzumab and all HER2-targeted therapy was 58.9 months (27.6–88.1) and 69.1 months (35.6–124.5), respectively. 85% of LTS had a period off HER2 therapy, lasting a median of 30.4 months (8.2–NR).
Conclusions
LTS generally receive HER2-targeted therapies for periods of time longer than in clinical trials, but most LTS also had breaks in treatment. More research is needed to understand the effects of long-term treatment and to identify patients who may be able to safely discontinue HER2-targeted therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development and routine use of trastuzumab and other HER2-targeted therapies mean that the typical person diagnosed with HER2-positive metastatic breast cancer (HER2+MBC) today lives longer than most people diagnosed with HER2-negative MBC [1]. Survival estimates from clinical trials have been increasing over time; the original pivotal trial of trastuzumab combined with chemotherapy reported a median overall survival (OS) of 25.1 months [2], and the more recent CLEOPATRA trial of first-line pertuzumab, trastuzumab, and chemotherapy reported median survival of 56.5 months [3].
Despite these recognized improvements in survival observed in clinical trials and routine clinical care [4], there are little data on the experience of patients surviving beyond median survival estimates. Most contemporary trials report estimates based on follow-up times around 4 years or less [3] and observational studies have focussed on the clinicopathological factors associated with long-term response/survival [5,6,7,8,9,10,11,12,13,14]. Understanding the experience of long-term survivors (LTS) is important for clinicians, patients, and health policy makers.
Many patients have questions about their likely survival time when diagnosed with HER2+MBC. Providing patients with median survival estimates from clinical trials may have little meaning for individuals several years into treatment and these estimates do not convey information on the likelihood of longer survival or what patients may experience from longer-term therapy. Moreover, trials do not provide information on the experience of typical patients treated in routine clinical care nor do they have the capacity to identify what typical patients experience in terms of durations and time off therapy. While current guidelines recommend continuing HER2-targeted therapy for as long as is feasible [15], it remains unknown if HER2-targeted therapies can be safely ceased after a period of disease stability; observational studies have the capacity to examine how this is being managed in contemporary practice.
Therefore, we sought to describe the real-world treatment patterns and survival outcomes for HER2+MBC patients surviving 5 years or longer from the start of treatment with trastuzumab for HER2+MBC. We calculate the proportion of patients initiating trastuzumab for HER2+MBC surviving for at least 5-years and estimate their: conditional probability of surviving an additional 5 years; time on trastuzumab and other HER2-targeted therapies; frequency and duration of breaks from trastuzumab and other HER2-targeted therapies; and time on additional cancer therapies.
Data sources and methods
Setting and data
The Australian healthcare setting and datasets used in this study have been described previously [16]. Briefly, Australia maintains a publicly funded, universal healthcare system entitling all citizens and permanent residents to subsidized medicines through the Pharmaceutical Benefits Scheme (PBS). The Herceptin Program, separate to the PBS, provided fully subsidized access to trastuzumab for HER2+MBC from December 2001 until July 2015, when the program was closed and trastuzumab for HER2+MBC was listed for subsidy on the PBS [16]. Australia has a single-payer healthcare system. Once a medicine is subsidized through the PBS or the Herceptin Program, the government bears the cost of the medicine. Private insurance will not provide reimbursement for publicly subsidized medicines. Patients can elect to pay out-of-pocket for medicines but given the high cost of trastuzumab, self-funding would be extremely unlikely. As such, our study population likely captures all Australians receiving trastuzumab for MBC during the study period.
The Australian Department of Human Services (DHS)—administering body for the Herceptin Program and PBS—supplied de-identified, patient-level data including patient information (year of birth, month/year of death, patient weight at enrolment, and HER2 immunohistochemistry (IHC) or in situ hybridization (ISH) result); dispensed trastuzumab records (dates and quantity supplied); and PBS dispensing records (all other prescription medicines). The DHS also provided the dispensing records for all patients in Australia who accessed publicly subsidized trastuzumab for early breast cancer (EBC) from 1 October 2006 to 30 June 2016. We determined previous treatment with trastuzumab for EBC through data linkage of Herceptin Program records with the dispensing records of patients who received trastuzumab for EBC.
The period of time observed across the datasets is 1 January 2001 to 30 June 2016.
Study design and participants
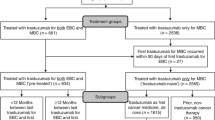
Our population-based, retrospective cohort study includes every Australian woman initiating trastuzumab for MBC subsidized through the Herceptin Program between 3 December 2001 and 30 June 2011 to allow for a minimum of 5 years potential observation time for each patient. All patients were observed until death or 30 June 2016.
Outcomes and statistical analysis
We used descriptive statistics to summarize age, weight, fact of death, determination of HER2+ (IHC or ISH), and the number of patients dispensed endocrine therapies.
We estimated: OS from the time of first trastuzumab dispensing for HER2+MBC date until month of death (set at the last day of the month) or censor using Kaplan–Meier methods; conditional probability of surviving to 10 years given a patient survived five according to Hieke et al [17]; time on each HER2-targeted therapy for HER2+MBC as the period from first dispensing date until the last dispensing date, plus 30 days or the number of days to death, whichever was sooner (we considered a period of > 90 days between dispensings as a break in treatment and a dispensing following a break of > 90 days as beginning a new course of therapy) [18]; and time on and off trastuzumab and all HER2-targeted therapies using Kaplan–Meier methods. To account for variable survival times and patients’ opportunities to receive treatments, we used estimates of duration and survival to determine the proportion of observed survival time that patients spent on: trastuzumab, all HER2-targeted therapies, and chemotherapy.
We used PBS dispensing data to summarize the type, number, and timing of other cancer treatments dispensed following trastuzumab initiation for HER2+MBC. We determined first-line partner therapy based on treatments dispensed during the period from 30 days prior to 90 days following trastuzumab initiation. We calculated the time on other cancer therapies in the same manner as for HER2-targeted therapies, and summarized the number of unique chemotherapies dispensed following trastuzumab initiation.
All analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC).
Ethics and data access approvals
Our study was approved by the NSW Population and Health Services Research Ethics Committee (Approval Number: 2010/02/213) and data access was granted by the Australian Department of Human Services (DHS) External Request Evaluation Committee (Approval Numbers: MI1474, MI1475, MI1477, MI5858). Individual consent for the release of these data has been waived according to the Australian Privacy Act of 1988 [16]. Access to the datasets analyzed during the current study is not permitted without the express permission of the approving human research ethics committees and data custodians.
Results
Patient characteristics
There were 4177 women initiating trastuzumab for HER2+MBC between 3 December 2001 and 30 June 2011. Their median OS was 28.1 months (IQR 12.6–62.9; Fig. 1) and 1082 women (26%) survived at least 5 years from their first trastuzumab dispensing for HER2+MBC. These LTS had a median age of 54 years (IQR 46–63; Table 1); 60 (6%) received prior adjuvant trastuzumab; and most (65%) were also dispensed at least one endocrine therapy either prior to initiation of trastuzumab for HER2+MBC or within the first 5 years of treatment. Of the 443,148 vials of trastuzumab dispensed to all patients initiating trastuzumab during the study period, 240,169 (54%) were dispensed to LTS (Table 1).
Overall survival and duration of HER2-targeted therapies
By 30 June 2016, 36% of all LTS had died. Median OS from year 5 of treatment was 78.9 months (IQR 23.3–not reached; Fig. 2) and the conditional probability of surviving an additional 5 years from this time was 55%. Median time on trastuzumab from commencement of treatment for HER+MBC, excluding breaks, was 58.9 months (IQR 27.6–88.1; Table 2) and median duration of the first course of trastuzumab was 40.3 months (IQR 14.1–73.6). Non-trastuzumab HER2-targeted therapies were dispensed to 333 LTS (31%) following trastuzumab initiation: 246 (23%) received lapatinib, 111 (10%) received trastuzumab emtansine (T-DM1), and 18 (2%) received pertuzumab (Table 1). The median time on all HER2-targeted therapy was 69.1 months (IQR 35.6–124.5) and the median proportion of survival time spent on HER2-targeted therapies was 83% (IQR 39–97%; Table 2).
Chemotherapy and endocrine therapy
Chemotherapy was dispensed to 909 (84%) LTS and the median proportion of OS time spent on chemotherapy was 13% (5–31%; Table 2). Taxanes were the most common first-line treatment dispensed with trastuzumab (54% of LTS). There were 173 (16%) LTS who did not receive chemotherapy during or following trastuzumab—96 received endocrine therapies while 77 received only trastuzumab. Capecitabine, lapatinib, vinorelbine, and gemcitabine were most often dispensed between the 4th and 6th years from trastuzumab initiation. Beyond year 5, the most commonly dispensed medicines were endocrine therapies and taxanes.
Trastuzumab cessation and re-starting among long-term survivors
Of the 617 LTS with only one course of trastuzumab therapy, 115 (19%) received other HER2-targeted therapies, 153 (25%) received endocrine therapies, and 127 (21%) received chemotherapy following trastuzumab cessation; 180 (29%) continued on the first course until death or censor. Among the 465 patients with more than one course of trastuzumab, the first trastuzumab course ceased most often after 1 year following treatment initiation. The second trastuzumab course most often commenced between 1 and 2 years following initiation of treatment, though the timing of cessation and re-commencing treatment were fairly constant between years 1 and 5 of treatment (Supplementary Figure A). The median time from the cessation of the first course of trastuzumab to the start of the second course was 5.7 months (IQR 2.7–17.1; Table 2).
Time off treatment
Most LTS (83%) had a period of time off HER2-targeted therapies, regardless of whether they re-started treatment later. The median time off HER2-targeted therapies was 30.4 months (IQR 8.2–not reached; Table 2). During periods off HER2-targeted treatment, patients were most often dispensed capecitabine (15%), endocrine therapies (14%), and taxanes (10%). Most LTS (78%) also had a period of time during which they received no cancer medicines at all, lasting a median duration of 14.2 months (IQR 3.7–not reached).
Additionally, there were 280 (26%) LTS who spent more than 50% of the first 5 years from trastuzumab initiation off HER2-targeted therapy. The majority of these LTS (69%) received one course of trastuzumab lasting a median of 13.3 months (IQR 8.4–16.7), and median time on all HER2-targeted therapies was 14.9 months (IQR 12.4–22.2). While off HER2-targeted therapies, the most common dispensed treatments included endocrine therapies (12%), taxanes (7%), capecitabine (7%), but 84% of these LTS received no cancer treatments when off HER2-targeted therapy. All but 12 of these patients remained off HER2-targeted therapies for > 50% of their observed survival time.
Discussion
In this population-based cohort study, we describe the largest cohort of LTS with HER2+MBC treated with trastuzumab in the literature to date. We found 26% of women initiating trastuzumab for MBC lived five or more years. Several observational studies [5,6,7,8,9,10,11,12,13,14] have examined clinicopathological factors associated with long-term response/survival in institution-based HER2+MBC cohorts, but they did not examine treatment patterns over the long-term; our study provides these useful data for patients, clinicians, and policy makers.
Questions around the acceptability of discontinuing trastuzumab and other HER2-targeted therapies beyond a specific time point remain particularly relevant. Witzel et al. [9] found that interrupting trastuzumab was associated with significantly shorter progression-free survival (PFS), while Murthy et al. [6] reported data for three patients with OS times longer than 10 years who had been off HER2-targeted therapy for over 9 years. We observed that most LTS experience periods of time off trastuzumab and other HER2-targeted therapies, and that the estimated median time off all HER2-targeted therapy was over 2 years. Moreover, there were 280 LTS who spent the majority of their survival time off HER2-targeted therapy. We were unable to adjust for clinical confounders, but our results suggest that some patients may be able to stop HER2-targeted therapies and still achieve long survival.
While LTS experienced breaks in treatment, they were treated for periods far longer than in clinical trials. The median duration of trastuzumab therapy was 5 years and LTS accounted for the majority (54%) of trastuzumab dispensed during the study period, despite comprising 26% of treated patients. While the long survival times are good news for patients, the extended treatment periods are unprecedented for cancer medicines outside of endocrine therapies, and would not have been anticipated at the time that trastuzumab was approved for public subsidy in Australia. This finding highlights the importance of this type of observational, post-market research. The funding of HER2-targeted and other targeted cancer therapies is a major challenge facing current policy makers and medicine funding bodies [19, 20] and these inputs can help inform decisions around funding future targeted cancer therapies.
These extended treatment periods also emphasize the need for more research on the impact of long-term, cumulative exposure to these medicines. The uncertainties surrounding safety and continued efficacy associated with years of continuous treatment with HER2-targeted therapies highlight the need to identify the patients for whom these medicines might safely be discontinued, as well as the need for a better understanding of the mechanisms of action for these medicines [21]. If continued blockade of the HER2/3 receptors drives the survival gains associated with these medicines, then continuous treatment is required. However, if efficacy is driven more by antibody-dependent cytotoxicity, there may be a point by which continued treatment does not provide additional survival benefit and may unnecessarily expose patients to treatment-related risks, such as cardiotoxicity as well as the inconvenience of receiving treatment every 3 weeks.
Currently, it is not possible to pinpoint the characteristics of patients who are likely to be LTS, nor do we know how long to continue trastuzumab therapy in patients with stable disease. Several observational studies have investigated clinicopathological factors associated with long-term survival and/or prolonged response to treatment [5,6,7,8,9,10,11,12,13,14]. Because no consensual definition of “long-term survival/response” exists, these studies used a variety of outcome measures to define their cohorts: at least 5 years overall survival;[5, 6, 12] greater than 1–3 years of progression-free survival;[7, 9, 11, 14] duration of therapy;[8] and statistical methods;[10] and the reported results are similarly heterogeneous. Broadly, the clinical characteristics associated with long-term survivors/responders in these studies included: better performance status, locoregional recurrences as opposed to distant metastases, fewer metastatic sites, and younger age at trastuzumab initiation. Measures such as hormone receptor positivity and no prior adjuvant trastuzumab have been shown to be significantly associated with long-term survival/response in some studies [6, 8, 10, 14] but not others [5, 7, 9, 11]. One study examining molecular markers found that genes related to the PI3K pathway were associated with poorer response to trastuzumab and shorter survival [14]. The heterogeneity in cohort definitions and generally small sample sizes of these studies make it difficult to characterize the current understanding of long-term survival/response in HER2+MBC patients. A consistent definition of “long-term survival/response” for HER2+MBC would facilitate a more cohesive dialogue around this unique patient sub-group and help in future work to discern the clinical factors and biomarkers that might identify LTS at the time of treatment initiation.
Comparing our study with such a diverse milieu of clinical and genetic studies is challenging, but three studies [5, 6, 12] used the same 5-year survival criterion applied in our study to define long-term survival. The proportion of patients surviving to 5 years in our population-based cohort (26%) is similar to those reported from these institution-based studies (14.5, 33, and 31%, respectively). A longer 5-year survival rate (54%) was reported in another recent dual-institution study; however, all 483 patients had de novo HER2+MBC and 20% received pertuzumab in addition to trastuzumab [13]. The only study to estimate median OS for patients surviving at least 5 years was that by Harano et al. [5], where the median OS for 5-year LTS was 92.2 months. This was shorter than our estimate of 138.9 months. Reasons for this discrepancy may include differences in the sites and extent of metastases, treatments received, and the more recent time period for our study (2001–2016 as opposed to 1994–2012). Previous research using our data demonstrated that median OS increased with time between 2001 and 2015 [4].
The most recent HER2-targeted therapies—pertuzumab and T-DM1—became available in Australia from July 2015 and few LTS in our study received pertuzumab (2%) or T-DM1 (10%). With the availability of these new therapies, it is likely that survival times for HER2+MBC patients diagnosed today will be longer than observed in our study. Previous trastuzumab treatment for early breast cancer (EBC) may be an important prognostic factor for patients initiating trastuzumab for MBC [22], but adjuvant trastuzumab was not subsidized in Australia until October 2006 and most patients in our study did not receive adjuvant trastuzumab.
The data for our study were collected for reimbursement and a lack of clinical information is their primary limitation. We are unable to describe the clinical or molecular characteristics of our LTS and how these factors might differ from other patients initiating trastuzumab during the study period. We do not have data on date of MBC diagnosis, performance status and extent of disease. We cannot determine how many of the LTS in our study had locoregional disease versus distant metastases. Similarly, we do not know if the tumor tested for HER2-amplification was from the primary tumor, or from a metastatic site. The strengths of this study include the size and representativeness of the national sample, as well as 14.5 years of observation time. Our cohort was selected from all women treated with publicly funded trastuzumab for HER2+MBC in Australia, which, given the high cost of trastuzumab, likely represents the vast majority of Australian women treated during the study period.
Conclusions
Our results provide generalizable estimates of OS, time on and off treatment, as well as the proportion of patients initiating HER2-targeted therapy that may expect to survive at least 5 years. HER2+MBC patients are living longer and with the availability of pertuzumab and T-DM1 survival times are likely to increase further. It will be many years before data are available allowing examination of long-term outcomes associated with their use and in the absence of these data our study provides useful baseline information. Our study highlights the fact that some patients can discontinue trastuzumab treatment, but more research is needed to understand who might be able to do so, and when they might be able to do it. Our results also emphasize the fact that patients can and do stay on HER2-targeted therapies for periods of time far beyond clinical trial estimates. Policy makers will need to take this into account when making subsidy decisions for new targeted therapies.
References
Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH (2010) Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol 28(1):92–98. https://doi.org/10.1200/JCO.2008.19.9844
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792. https://doi.org/10.1056/NEJM200103153441101
Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, Clark E, Ross G, Benyunes MC, Cortes J, Group CS (2015) Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372(8):724–734. https://doi.org/10.1056/NEJMoa1413513
Daniels B, Kiely BE, Lord SJ, Houssami N, Lu CY, Ward RL, Pearson S-A (2017) Trastuzumab for metastatic breast cancer: Real world outcomes from an Australian whole-of-population cohort (2001–2016). Breast 38:7–13. https://doi.org/10.1016/j.breast.2017.11.007
Harano K, Lei X, Gonzalez-Angulo AM, Murthy RK, Valero V, Mittendorf EA, Ueno NT, Hortobagyi GN, Chavez-MacGregor M (2016) Clinicopathological and surgical factors associated with long-term survival in patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat 159(2):367–374. https://doi.org/10.1007/s10549-016-3933-6
Murthy P, Kidwell KM, Schott AF, Merajver SD, Griggs JJ, Smerage JD, Van Poznak CH, Wicha MS, Hayes DF, Henry NL (2016) Clinical predictors of long-term survival in HER2-positive metastatic breast cancer. Breast Cancer Res Treat 155(3):589–595. https://doi.org/10.1007/s10549-016-3705-3
Spano JP, Beuzeboc P, Coeffic D, Arnould L, Lortholary A, Andre F, Ferrero JM (2015) Long term HER2 + metastatic breast cancer survivors treated by trastuzumab: results from the French cohort study LHORA. Breast 24(4):376–383. https://doi.org/10.1016/j.breast.2015.02.035
Vaz-Luis I, Seah D, Olson EM, Wagle N, Metzger-Filho O, Sohl J, Litsas G, Burstein HJ, Krop IE, Winer EP, Lin NU (2013) Clinicopathological features among patients with advanced human epidermal growth factor-2-positive breast cancer with prolonged clinical benefit to first-line trastuzumab-based therapy: a retrospective cohort study. Clin Breast Cancer 13(4):254–263. https://doi.org/10.1016/j.clbc.2013.02.010
Witzel I, Muller V, Abenhardt W, Kaufmann M, Schoenegg W, Schneeweis A, Janicke F (2014) Long-term tumor remission under trastuzumab treatment for HER2 positive metastatic breast cancer—results from the HER-OS patient registry. BMC Cancer 14:806. https://doi.org/10.1186/1471-2407-14-806
Yardley DA, Tripathy D, Brufsky AM, Rugo HS, Kaufman PA, Mayer M, Magidson J, Yoo B, Quah C, Ulcickas Yood M (2014) Long-term survivor characteristics in HER2-positive metastatic breast cancer from registHER. Br J Cancer 110(11):2756–2764. https://doi.org/10.1038/bjc.2014.174
Yeo B, Kotsori K, Mohammed K, Walsh G, Smith IE (2015) Long-term outcome of HER2 positive metastatic breast cancer patients treated with first-line trastuzumab. Breast 24(6):751–757. https://doi.org/10.1016/j.breast.2015.09.008
Steenbruggen T, van Ramshorst M, Stouthard J, Rodenhuis S, Linn S, Sonke G, Smorenburg C (2017) Abstract P4-21-30: long-term survival in HER2-positive metastatic breast cancer: the first blow is half the battle. Can Res 77 (4 Suppl):P4-21-30. https://doi.org/10.1158/1538-7445.sabcs16-p4-21-30
Wong Y, Raghavendra AS, Hatzis C, Irizarry JP, Vega T, Barcenas CH, Gregor MC-M, Valero V, Tripathy D, Pusztai L, Murthy RK (2017) Long-term survival of de novo stage IV human epidermal growth factor receptor 2 (HER2)-positive breast cancers treated with HER2 targeted therapy. J Clin Oncol 35(15_suppl):1021–1021. https://doi.org/10.1200/JCO.2017.35.15_suppl.1021
Gamez-Pozo A, Perez Carrion RM, Manso L, Crespo C, Mendiola C, Lopez-Vacas R, Berges-Soria J, Lopez IA, Margeli M, Calero JL, Farre XG, Santaballa A, Ciruelos EM, Afonso R, Lao J, Catalan G, Gallego JV, Lopez JM, Bofill FJ, Borrego MR, Espinosa E, Vara JA, Zamora P (2014) The Long-HER study: clinical and molecular analysis of patients with HER2+ advanced breast cancer who become long-term survivors with trastuzumab-based therapy. PLoS ONE 9(10):e109611. https://doi.org/10.1371/journal.pone.0109611
Cardoso F, Costa A, Senkus E, Aapro M, Andre F, Barrios CH, Bergh J, Bhattacharyya G, Biganzoli L, Cardoso MJ, Carey L, Corneliussen-James D, Curigliano G, Dieras V, El Saghir N, Eniu A, Fallowfield L, Fenech D, Francis P, Gelmon K, Gennari A, Harbeck N, Hudis C, Kaufman B, Krop I, Mayer M, Meijer H, Mertz S, Ohno S, Pagani O, Papadopoulos E, Peccatori F, Penault-Llorca F, Piccart MJ, Pierga JY, Rugo H, Shockney L, Sledge G, Swain S, Thomssen C, Tutt A, Vorobiof D, Xu B, Norton L, Winer E (2017) 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol. https://doi.org/10.1093/annonc/mdx036
Daniels B, Lord SJ, Kiely BE, Houssami N, Haywood P, Lu CY, Ward RL, Pearson S-A (2017) Use and outcomes of targeted therapies in early and metastatic HER2-positive breast cancer in Australia: protocol detailing observations in a whole of population cohort. BMJ Open 7(1). https://doi.org/10.1136/bmjopen-2016-014439
Hieke S, Kleber M, Konig C, Engelhardt M, Schumacher M (2015) Conditional survival: a useful concept to provide information on how prognosis evolves over time. Clin Cancer Res 21(7):1530–1536. https://doi.org/10.1158/1078-0432.CCR-14-2154
Pearson SA, Ringland CL, Ward RL (2007) Trastuzumab and metastatic breast cancer: trastuzumab use in Australia–monitoring the effect of an expensive medicine access program. J Clin Oncol 25(24):3688–3693. https://doi.org/10.1200/jco.2007.11.2516
Vitry A, Mintzes B, Lipworth W (2016) Access to new cancer medicines in Australia: dispelling the myths and informing a public debate. J Pharm Policy Pract 9(1):13. https://doi.org/10.1186/s40545-016-0062-x
Karikios DJ, Schofield D, Salkeld G, Mann KP, Trotman J, Stockler MR (2014) Rising cost of anticancer drugs in Australia. Intern Med J 44(5):458–463. https://doi.org/10.1111/imj.12399
Vu T, Claret FX (2012) Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol 2:62. https://doi.org/10.3389/fonc.2012.00062
Daniels B, Kiely BE, Houssami N, Lord SJ, Dobbins T, Lu CY, Ward RL, Pearson SA (2017) Survival outcomes for Australian women receiving trastuzumab for HER2-positive metastatic breast cancer following (neo)adjuvant trastuzumab: a national population-based observational study (2006–2014). Br J Cancer. https://doi.org/10.1038/bjc.2017.405
Acknowledgements
We acknowledge the contribution of Sally Crossing (AM) (1946–2016) as the Health Consumer Advocate on this research program. We thank the Department of Human Services for providing the data for this research.
Funding
This work was supported by a Cancer Australia Priority Driven Collaborative Support Scheme (ID: 1050648) and the NHMRC Centre of Research Excellence in Medicines and Ageing (CREMA; ID: 1060407). BD is supported by an NHMRC Postgraduate Research Scholarship (ID: 1094325), the Sydney Catalyst Translational Cancer Research Centre (no grant number), and a CREMA PhD scholarship top-up (no grant number). NH receives funding through a National Breast Cancer Foundation (Australia) Breast Cancer Research Leadership Fellowship (no grant number).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
BEK has received conference support and a speaker’s honorarium from Roche. RLW is a member of the Pharmaceutical Benefits Advisory Committee (PBAC) and SAP is a member of the Drug Utilisation Sub Committee of the PBAC. The views expressed in this paper do not represent those of the either committee. The remaining authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Daniels, B., Kiely, B.E., Lord, S.J. et al. Long-term survival in trastuzumab-treated patients with HER2-positive metastatic breast cancer: real-world outcomes and treatment patterns in a whole-of-population Australian cohort (2001–2016). Breast Cancer Res Treat 171, 151–159 (2018). https://doi.org/10.1007/s10549-018-4804-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4804-0